The three-year epidemic has made most people more or less confused about the future, and a small number of people have been completely changed by the epidemic! After all, the epidemic will fade away with time. There may not be many traces of the past ravages, but I believe that some indelible things have been deeply imprinted in the minds of many people! What can really save us, apart from the mutual support and mutual warmth between people, may be the only vaccines and medicines brought about through scientific innovation! Especially the cutting-edge field of biomedicine may be the inevitable path for future human beings to open the door to the future! This industry, which is closely related to life and health, is also the most worthwhile investment industry in the capital market!
Let’s take a look at the evolution of the biomedical field in the past few years. Only by understanding the history can we find the basis for predicting the future!
As early as 1868, people had already discovered two kinds of nucleic acids – DNA and RNA , but at that time people were more inclined to think of proteins as genetic material. Until 1953, the British “Nature” magazine published Watson and Crick’s article “The Molecular Structure of Nucleic Acid – The Structure of DNA”. The discovery of the double helix structure of DNA has made people realize that it is the difference in the operation trajectory of DNA and genes that leads to the difference in the process of biological evolution and life. In 1958, Crick proposed the central dogma of molecules, indicating that the flow direction of genetic information is from DNA to RNA, and then from RNA to protein.
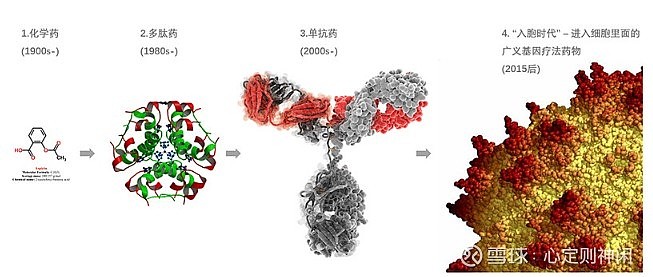
In the 1970s, in 1973, two scientists, Cohen and Boyle, successfully integrated the plasmid containing the Xenopus gene into E. coli, completing the first DNA cutting and joining, and gene recombination technology was born. In 1978, Genentech synthesized recombinant human insulin. In 1975, Kohler and Milstein discovered hybridoma technology, which can produce monoclonal antibodies in large quantities by using hybridoma cells, thus unveiling the prelude to antibody engineering.
In the 1980s, the development of global biopharmaceuticals started to accelerate. After Genentech synthesized recombinant human insulin, Eli Lilly quickly came to the door to reach a cooperation. In 1982, the world’s first genetically recombinant human insulin was officially launched, which was the first genetically recombinant biological product approved by the FDA. In 1986, the biopharmaceutical industry was in full bloom. First, the humanized antibody technology was established, which overcomes many shortcomings of mouse-derived antibodies used in human treatment. In addition, a number of important drugs have also been launched this year. The first therapeutic monoclonal antibody drug was launched to prevent renal transplant rejection; in the same year, the first anti-tumor biotechnology drug α-interferon was launched for the treatment of leukemia, and the mass production of interferon was truly realized; A gene recombinant vaccine, Hepatitis B vaccine, was also launched this year. This is the first vaccine product based on the recombination of viral protein gene sequences.
In the 1990s, Rituximab (Rituximab), the first monoclonal antibody for the treatment of tumors, was launched in 1997, and the development of monoclonal antibody drugs entered another new stage. In the following two years, the US FDA successively approved 7 monoclonal antibodies and 1 TNF-α receptor inhibitory protein, and the global biopharmaceutical industry ushered in rapid development.
On June 26, 2000, scientists from the United States, Japan, the United Kingdom, France, Germany and China, who participated in the Human Genome Project, announced to the world the working draft of the human genome, deciphered 97% of the genetic code of the human body, and completed the The base pair sequencing of 85% of the genes laid the foundation for the final completion of all sequencing work. Since then, biopharmaceuticals have ushered in a prosperous era. During the 10 years from 2001 to 2010, the US FDA approved a total of 20 monoclonal antibody drugs. In 2010, five of the top 10 drugs sold globally were monoclonal antibody drugs. In 2010, the global sales of monoclonal antibody drugs reached 44 billion US dollars, and the momentum of development is rapid.
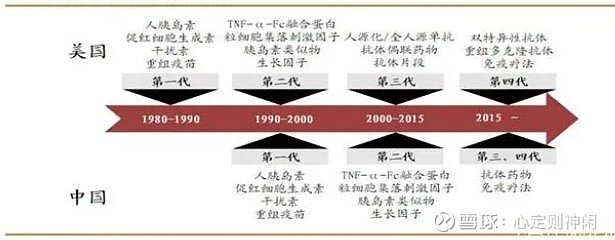
In China, before 2015, the research and development of biological drugs still basically stayed in the second stage of the development of biological drugs in Europe and the United States. From the perspective of approval of Class 1 biological drugs, most of them were recombinant proteins and vaccines. After 2015, with the introduction of a series of new drug policies, the influx of overseas capital, and the return of a large number of medical talents, domestic biopharmaceutical research and development finally ushered in a period of vigorous development. During this period, the research and development of biopharmaceuticals in my country directly skipped the development of the third phase, and proceeded with the simultaneous development of the third and fourth phases, and gradually narrowed the field of research hotspots such as CAR-T, PD-1 inhibitors and bispecific antibodies. The gap with Europe and the United States.
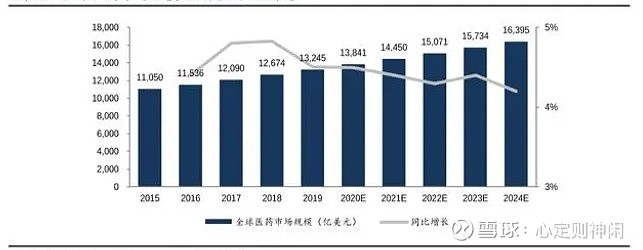
China’s biopharmaceutical market is still in its early stages of development, and the growth of the industry is also very strong. From the perspective of my country’s biological drug market structure: my country’s chemical drug market accounts for the largest share, with a market share of 43.7% in 2018; biological drugs accounted for 11.7%. As the structure of the pharmaceutical market continues to diverge, the proportion of the biopharmaceutical market will gradually expand in the future, reaching 12.5% according to preliminary statistics in 2019. From 2016 to 2020, the scale of China’s biopharmaceutical market has grown from 183.6 billion to 387 billion. From 2014 to 2019, the compound growth rate of my country’s biopharmaceutical market reached 22.4%, far exceeding the 8% compound growth rate of the global biopharmaceutical market during the same period. According to the above growth rate, in 2023, the scale of my country’s biopharmaceutical market will reach more than 600 billion. Not only for the pharmaceutical industry, but also for the capital market has brought a huge investment opportunity!
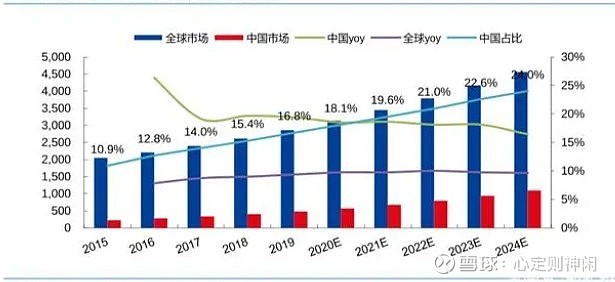
In 2018, among the top 10 drugs in global sales, there were 9 biopharmaceutical companies, contributing nearly 90% of the sales. However, due to the high barriers to R&D and production of biopharmaceuticals, domestic drug sales are still dominated by chemical drugs. In 2018, only two of the top ten drugs in sales in China were biopharmaceuticals, namely insulin aspart injection (NovoSharp) and insulin glargine injection (Lantus) for the treatment of diabetes. The global biopharmaceutical market is growing rapidly, and the growth rate is about twice that of the overall pharmaceutical market, far exceeding that of the chemical and traditional Chinese medicine markets. Among them, monoclonal antibody is the most mature and commercialized biopharmaceutical. In 2020, the global monoclonal antibody market will reach 181.9 billion US dollars, and the Chinese monoclonal antibody market will be about 44.8 billion yuan.
We have seen the past of the development of biomedicine clearly, but what are the future directions that deserve the most attention from our investors? From the perspective of investing in bio-innovative drugs, we should pay more attention to those R&D pipeline companies that have advantages in the hot track of biomedicine today. The three major fields of double antibody and ADC, genetic engineering, and nucleic acid drugs are the expected future in the field of biomedicine!
In the past year or so, our Chinese innovative drug companies have achieved a lot of fruitful results on the road to the international market. Rongchang Biotech’s new ADC drug Vidicumumab has joined hands with Seattle Gene and Akeso Bio for 2.6 billion US dollars. The Ak112 double-antibody 5 billion US dollars to join hands with Summit, 9 ADCs of Kelun Pharmaceuticals to authorize Merck & Co. to cooperate with more than 10 billion US dollars, etc. These achievements undoubtedly show that there are still many excellent innovative drug companies in China. The company is also our expectation for investing in the capital market in the new year!
Looking back at the various cold receptions that biomedical innovative companies have received in the capital market in the past two years, it is embarrassing! Especially in the Hong Kong stock market, those innovative drug and innovative medical device companies that once had incomparably brilliant and glorious moments are now doomed. Less than one-tenth of the original. These phenomena are obviously abnormal. When investors wake up from the abyss of emotion, it may bring about the rebirth of high-quality bio-innovative drug companies in the capital market! This time will not be too long, let us wish that in the new year, domestic biopharmaceutical companies will be able to scale new heights! $Kangfang Bio-B(09926)$ $Rongchang Biology(SH688331)$ $Kelun Pharmaceutical(SZ002422)$
Note: The above are just personal opinions and do not constitute any investment advice!
20230105 first published on the planet
There are 6 discussions on this topic in Xueqiu, click to view.
Snowball is an investor social network where smart investors are all here.
Click to download Xueqiu mobile client http://xueqiu.com/xz ]]>
This article is transferred from: http://xueqiu.com/2166236329/239760245
This site is only for collection, and the copyright belongs to the original author.