Original link: https://kaopubear.top/blog/2022-03-14-clinical-practice-gaps/
In other words, besides “expensive”, what other factors affect the application of precision cancer treatment in clinical practice?
A conversation that took place during Chinese New Year this year is as follows:
 : Young man, where do you get rich?
: Young man, where do you get rich?
 : Uh, I didn’t get rich.
: Uh, I didn’t get rich.
 : So what are you fussing about?
: So what are you fussing about?
 : In a big way, it is precision medicine, researching something that can detect genomic changes in cancer patients, and after using it, you can find precise treatment methods for patients.
: In a big way, it is precision medicine, researching something that can detect genomic changes in cancer patients, and after using it, you can find precise treatment methods for patients.
 : A very new thing?
: A very new thing?
 : Counting Laomei, the industry has been around for many, many years since its inception
: Counting Laomei, the industry has been around for many, many years since its inception
 : Then I heard that when people get cancer, why do they still use chemotherapy?
: Then I heard that when people get cancer, why do they still use chemotherapy?
 : It may be that this method of seeing a doctor has not really been popularized, and there are many irregularities in small places
: It may be that this method of seeing a doctor has not really been popularized, and there are many irregularities in small places
 : Then you are not researching for nothing?
: Then you are not researching for nothing?
 : Come, Uncle, have a drink
: Come, Uncle, have a drink
When I come back from the New Year, I will never forget this conversation. Apart from not knowing how to make a fortune, I am also curious about a question: so many upstream and downstream companies, including pharmaceutical companies with diagnostics, so many practitioners doing scientific research, R&D, and sales, After so many years, how many patients have benefited from it, and where should we look for problems?
In other words, apart from the “expensive” that you can think of with your eyes closed, what factors affect the application of precision cancer treatment in clinical practice?
After checking a few days ago, I really found a paper published in JCO Precision Oncology in October 2022.

Taking advanced non-small cell lung cancer (NSCLC) as an example, this paper introduces in detail the challenges of integrating predictive biomarker detection into the process of personalized cancer diagnosis and treatment, and quantifies the impact of various clinical practice barriers on The real impact of treatment.
Let me start with the conclusion: in the United States, approximately 64% of potentially beneficial advanced NSCLC patients did not benefit from cancer precision therapy appropriate for their disease due to patient losses caused by overlapping barriers in different clinical practices from diagnosis to treatment in the United States.
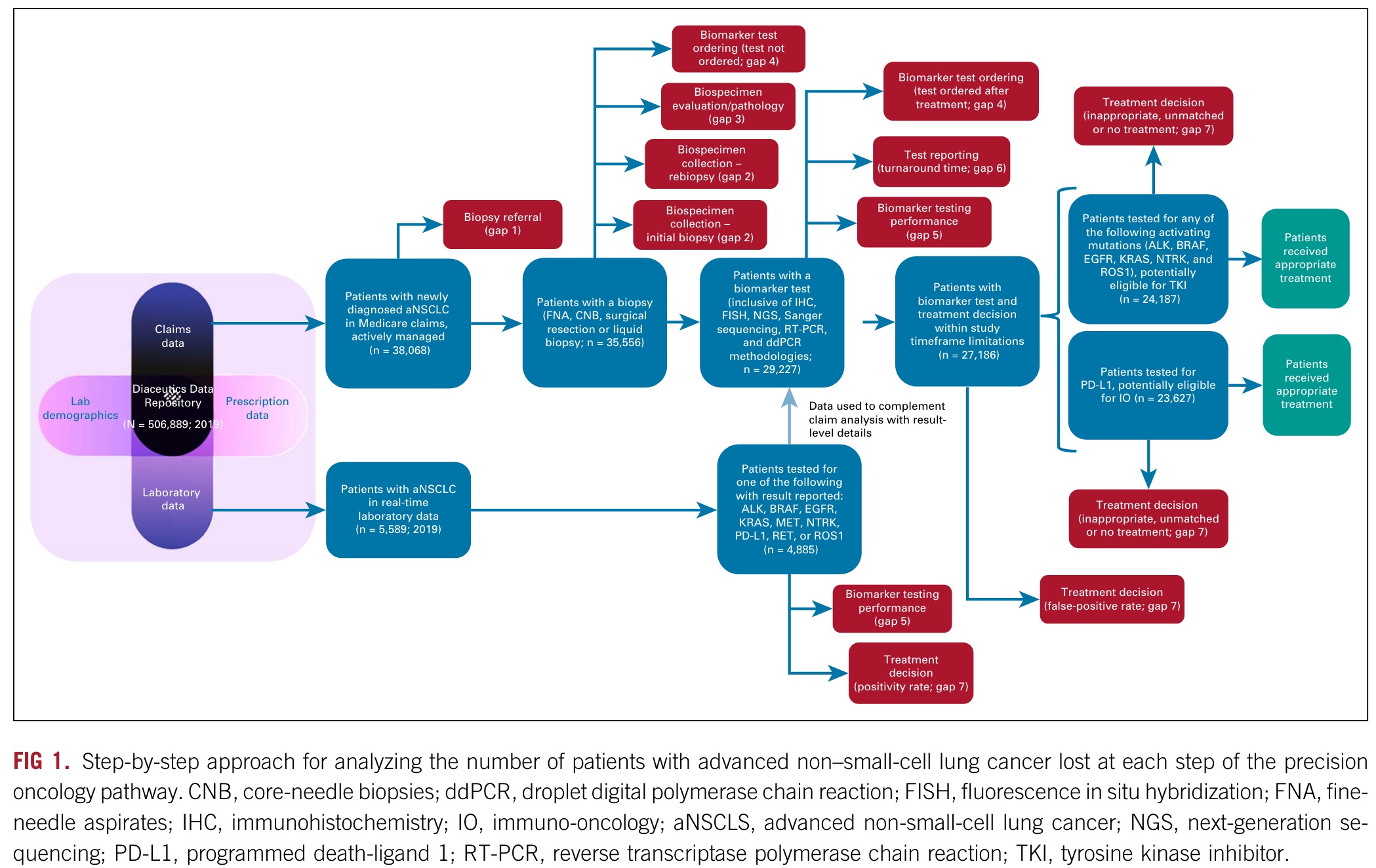
As shown in the figure above, Diaceutics used in the study is a multi-source database of data from more than 500,000 non-small cell lung cancer patients in the United States, including commercial and medical insurance and laboratory data.
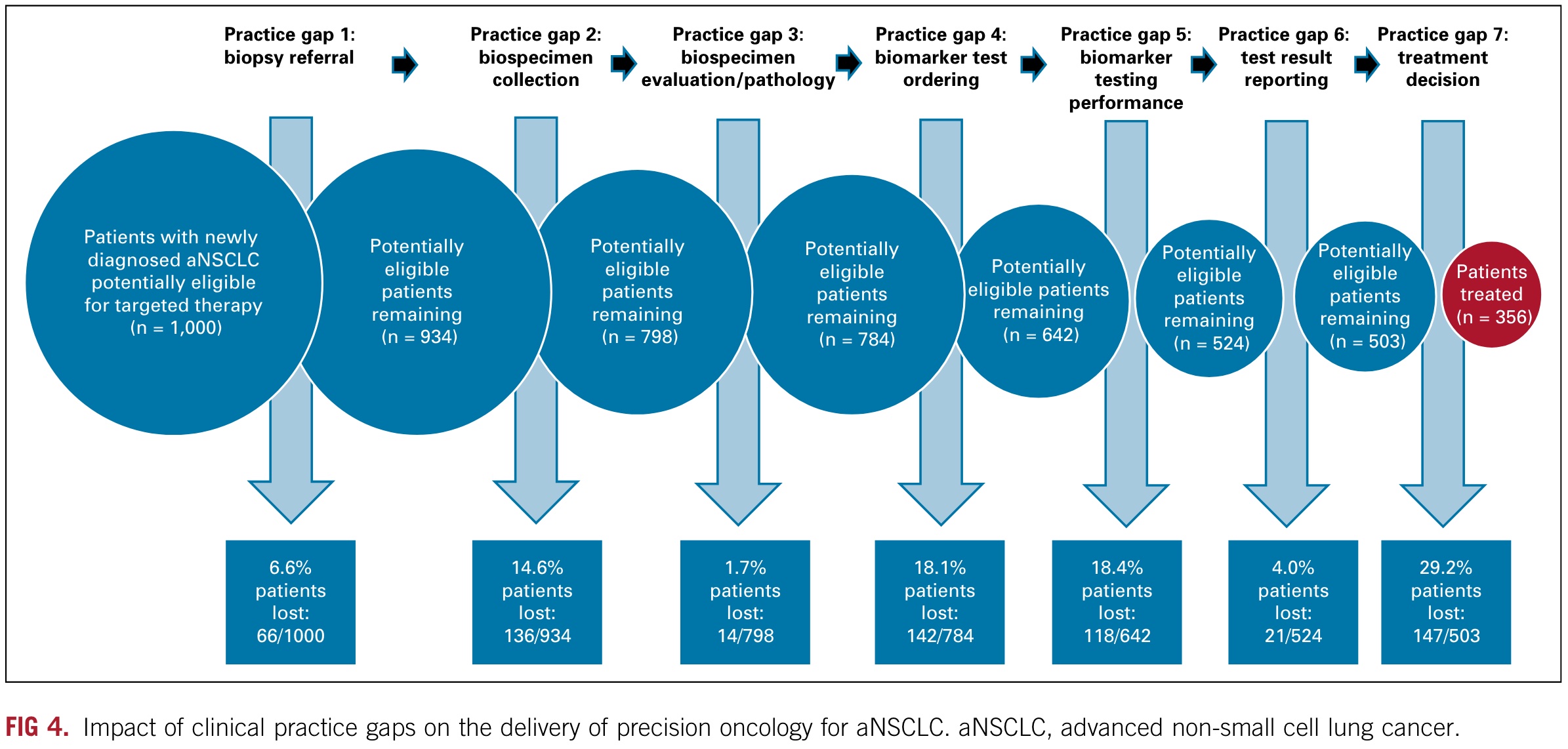
Its research focuses on the obstacles and their independent and cumulative effects in the seven steps from diagnosis to treatment in the precision treatment process.
The seven steps are as follows:
- Step 1: Biopsy referral: Initial solid or blood biopsy was never performed.
- Step 2: Biospecimen collection: Biospecimen collection challenges including insufficient tissue or tumor cell content of initial biopsy or rebiopsy inhibited biomarker testing and its accuracy.
- Step 3: Biospecimen evaluation/pathology: Biospecimen tumor cell content was overestimated, inhibiting biomarker testing and its accuracy. The overestimated tumor cell content affected biomarker detection and accuracy.
- Step 4: Biomarker test ordering: Appropriate testing was not ordered, or treatment began before testing was ordered.
- Step 5: Biomarker testing performance: Biomarker testing provided inconclusive or false-negative (FN) results. Biomarker testing provided inconclusive or false-negative (FN) results.
- Step 6: Test result reporting: As a result of turnaround time (TAT) delays, treatment was initiated without consideration of test results.
- Step 7: Treatment decision: Targeted treatment was not selected despite positive test results.
When the number of people is normalized to 1000, as shown in the figure below, you can see the specific problems in each step and the number and proportion of patients who have treatment opportunities in successive measures.
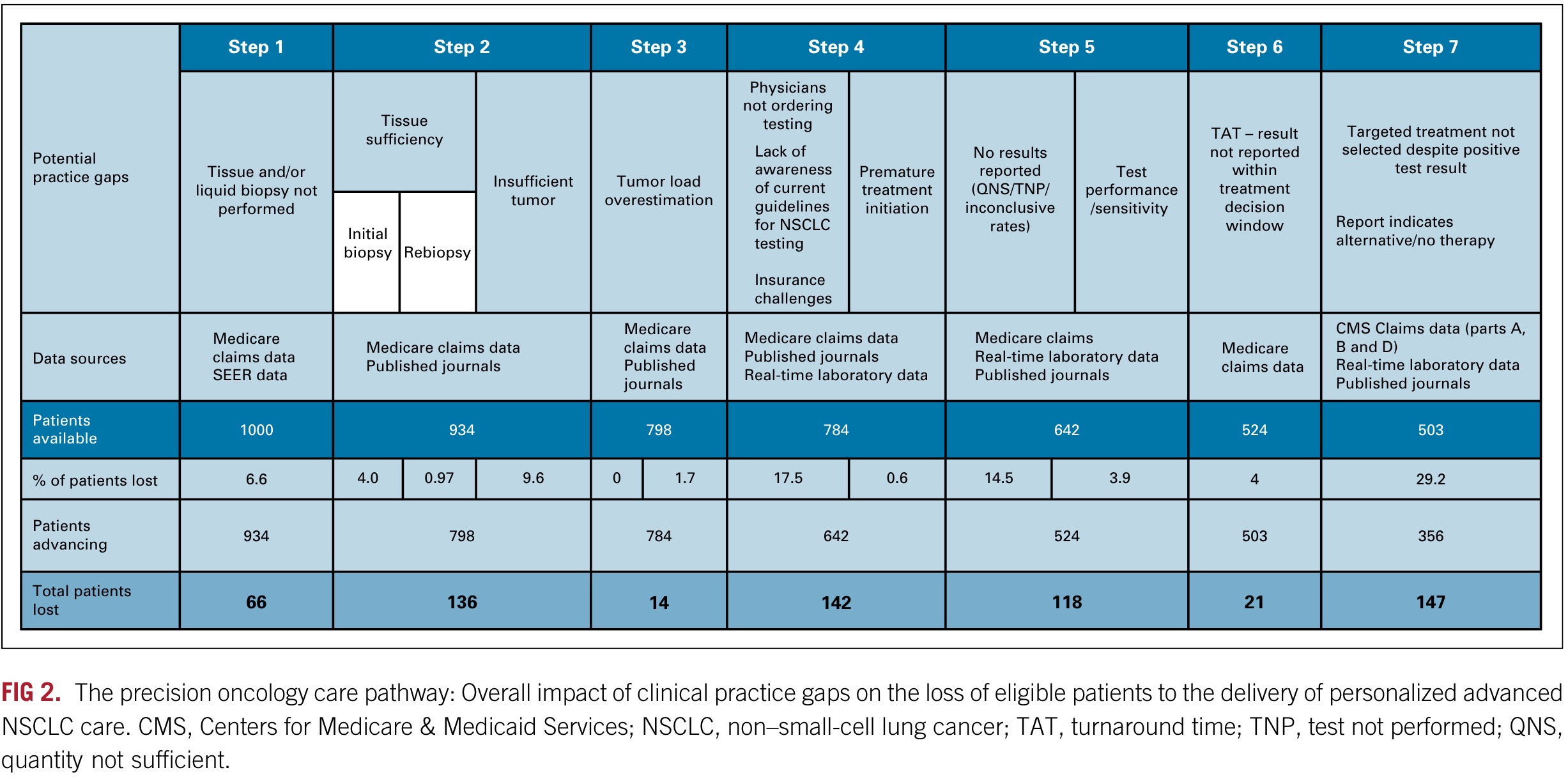
In the graph above, we can see that the researchers estimated that 29.2% of patients did not receive appropriate targeted treatment based on their test results.
Based on claims data, it was determined that 18.5% of patients were not treated. Of the 81.5% of patients who received various treatments, 9.1% received chemotherapy only, 14.3% received chemotherapy and immunotherapy, 40.8% received immunotherapy only, 16.6% received targeted therapy, and 0.7% received other treatments.
14.3% of tumors could have used TKI but did not receive the designated treatment. An additional 11.6% of patients had treatment-targetable results based on IHC testing but did not receive appropriate immunotherapy, and an estimated 3.3% of patients had incorrect test results (false positives).
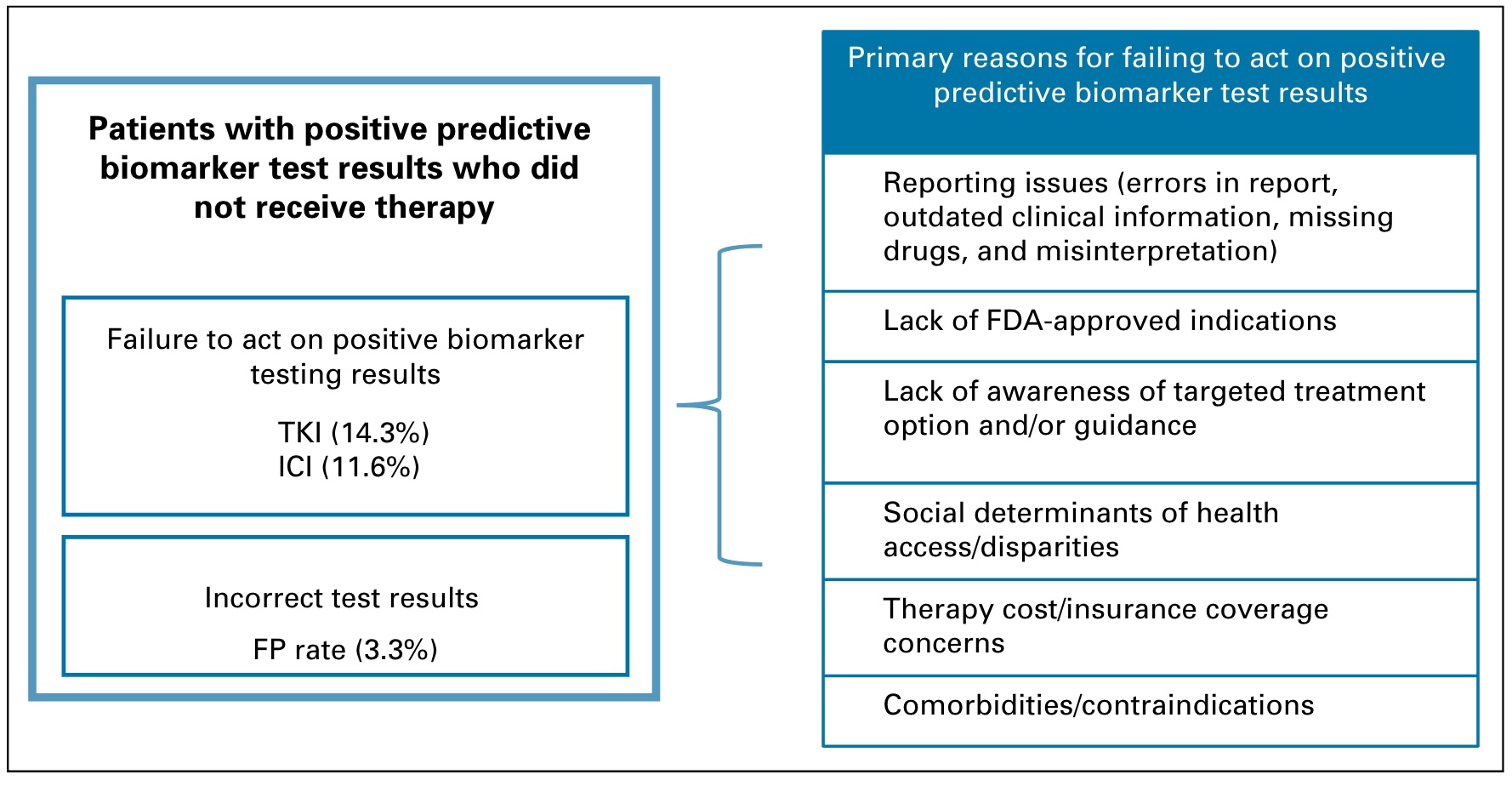
Speculated reasons for not receiving appropriate treatment, as shown in the figure below, may include: test reporting problems (errors in reports, outdated clinical and drug information); lack of FDA-approved indications (doctors are unclear or unwilling to use off-label drugs) ); lag in awareness of targeted therapy options; treatment accessibility; treatment cost/insurance coverage, etc.
If your focus is on tumor NGS testing, there is good news and bad news.
The bad news is the turnaround time (TAT) statistics: Of the 29,227 patients who underwent biomarker testing and reported the results, the study estimated that 4% experienced TAT delays that resulted in treatment decisions not taking molecular test results into account, 13.1% of all methods % of NGS assays for TAT exceeded 14 days.
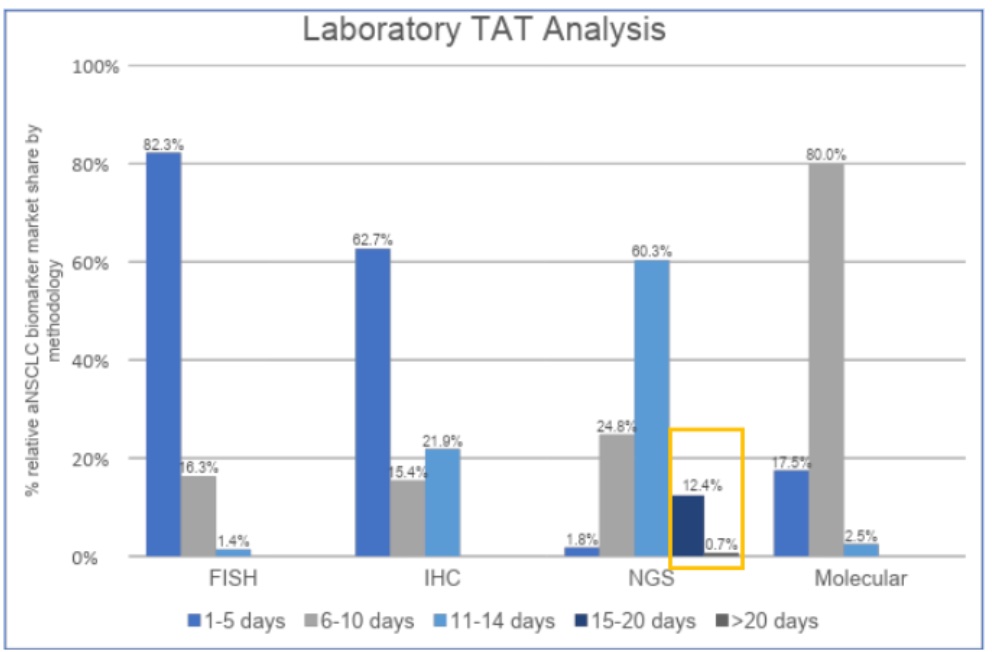
The good news is that in detection methods including PCR Sanger IHC and FISH, the FN of the NGS method is significantly smaller than other methods based on the false negative and false positive rates estimated by multiple studies.
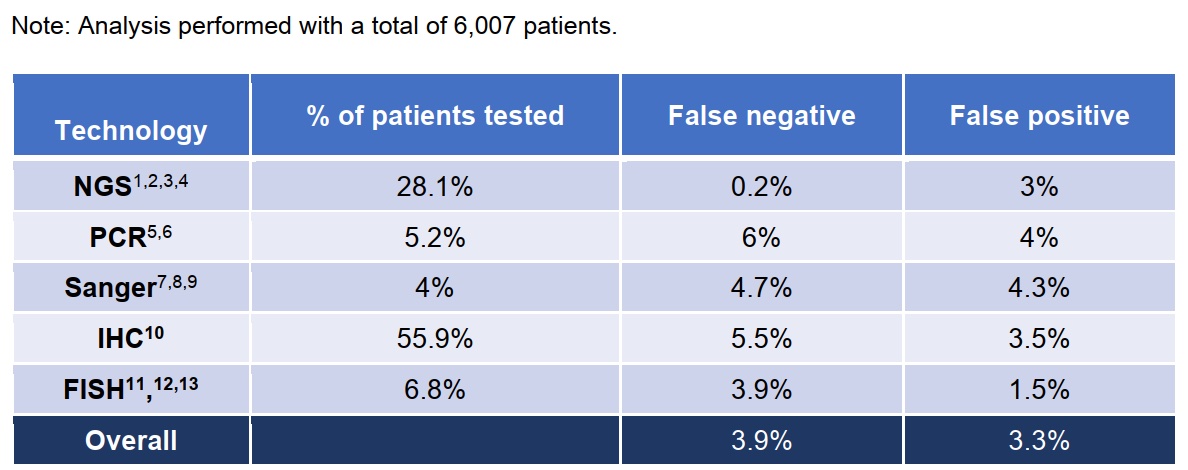
Seeing the last summary diagram of patient loss, I don’t know how you feel.
Problems represent opportunities and room for improvement, and the steps with larger problems are more likely to be optimized. However, in addition to paying attention to the effectiveness of the data (from 2019), you may also need to pay attention to the following points:
- This research is aimed at NSCLC, which is considered as a cancer type with a high chance of precision treatment in solid tumors. What about other cancer types?
- Among the seven clinical practice barriers, barrier seven has the highest patient loss rate in the United States, followed by barriers four and five. If similar research is conducted in China, which steps may be the most problematic?
- In the first step, 84.6% underwent tissue biopsy; 8.8% underwent liquid biopsy, and 6.6% of patients without tissue or liquid biopsy could be diagnosed by imaging only. From this data, what are the advantages of liquid biopsy compared with tissue biopsy, and how much space is there?
- In the third step, 38% of the estimated 14% of tissue samples with <\20% tumor cell content were overestimated as >20% and thus incorrectly considered suitable for molecular testing. In this part, in each tumor testing company, how many samples that should not be tested have been tested?
- For the above research, if a patient is considered to be tested multiple times along with the treatment, what will be the result?
- If there is still such a large proportion of potentially beneficial patients lost in pre-treatment (baseline) testing, what is the current situation of MRD and early screening that are competing for each other?
If you are interested in this article, please subscribe to my “Xiong Yan Xiong Yu” member newsletter via email or WeChat. I will share with you the latest industry research progress in the field of tumor biomedicine and what I think and learn. If you want , click this link to subscribe for free. 
This article is transferred from: https://kaopubear.top/blog/2022-03-14-clinical-practice-gaps/
This site is only for collection, and the copyright belongs to the original author.