Brain functions are fundamental to the survival of living organisms, and they are supported by neural circuits composed of various neurons. To study neuronal function at the single-cell level, researchers often use whole-cell patch-clamp recordings. These techniques allow us to record membrane potentials (including action potentials) of individual neurons in animals. This whole-cell recording approach allows us to reveal how neuronal activity supports brain function at the single-cell level. In this article, we present previous studies using in vivo patch-clamp recordings, as well as recent findings on behavioral function, primarily in the hippocampus. We further discuss how to bridge the gap between electrophysiology and biochemistry.
1 Introduction
The action potentials of a network of neurons are exquisite, each triggered by the spatiotemporal sum of synaptic inputs, a fundamental component of brain function. To study neural activity, researchers have developed various types of electrophysiological recording techniques, broadly divided into extracellular and intracellular methods. While extracellular recordings allowed us to obtain data on the dynamics of neuronal firing and collective oscillations generated by multiple cells surrounding the recording electrode, intracellular recordings allowed the measurement of subthreshold membrane potential dynamics and suprathreshold firing activity at the single-cell level (Fig. 1a). Based on the thickness of the recording electrode tip, these intracellular recordings are further divided into patch clamp and sharp electrode techniques. However, the higher impedance of sharp electrodes results in larger leakage currents and prevents voltage-clamp recordings. Patch-clamping is the only method capable of capturing the intracellular activity of individual neurons with a high signal-to-noise ratio.
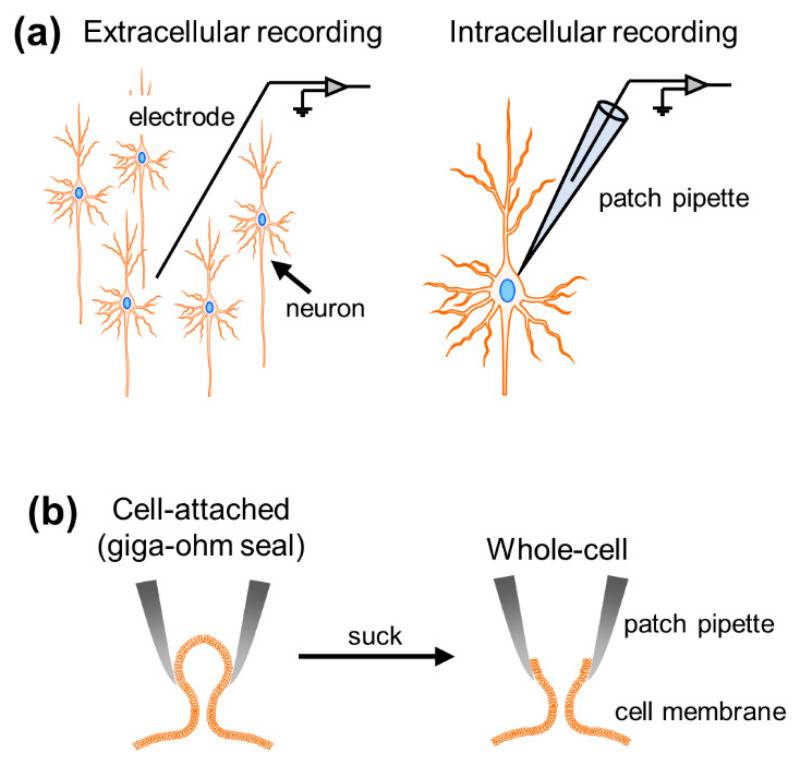
Figure 1 Overview of the patch clamp method. (a) Comparison between extracellular and intracellular recordings; (b) single-channel mode (left) and whole-cell mode (right).
To conduct patch-clamp experiments on neurons, researchers first need to draw glass electrodes (glass electrodes generally have a diameter of about 3µm and a resistance of 3-7MΩ). They then filled glass electrodes with artificial intracellular fluid and connected the electrodes to electrode holders in the recording device, into which AgCl-coated wires would be inserted that would touch the electrode fluid. After preparation, they pressed glass electrodes against the cell membrane and tightly sealed the membranes together with a resistance of >1 GΩ between the pipette and the membrane, called a gigaohm seal. This mode is also called “single channel mode” (Fig. 1b). This recording mode allows us to capture the dynamics of membrane currents generated by ions passing through ion channels on the cell membrane. Retrospectively, the patch-clamp method was originally developed to record single-channel currents. Once negative pressure is applied and a small hole is formed in the cell membrane (whole-cell mode), the net dynamics of whole-cell currents and voltages generated through all ion channels expressed on the cell membrane can be measured. Whole-cell currents reflecting synaptic inputs to neurons can be recorded by holding the voltage at a specific value (voltage clamp), while action potentials, or fluctuations in membrane potential below the discharge threshold, can be recorded by controlling the current clamped (current clamp). Although there are other recording modalities within the patch-clamp approach, this article will focus on whole-cell recording modalities in both voltage-clamp and current-clamp configurations.
2. In vivo whole-cell recording of anesthetized animals
2.1 Cerebral cortex
In vivo whole-cell recording was first performed in anesthetized animals. This is because establishing a stable GΩ seal is a critical process in whole-cell recordings, and achieving a high signal-to-noise ratio requires the brain to be as still as possible. Targeted areas in the early stages are the primary visual cortex (V1) and the primary somatosensory cortex (S1), as these cortices are closer to the outside of the brain and are more easily accessible than other cerebral cortical regions. When researchers clamp neurons, there is no actual field of view for neuron morphology and electrode position. This method of controlling electrodes without field of view is called “blind insertion”.
In 1994, whole-cell recordings were used to study the effect of GABA input on the direction selectivity of cat V1 pyramidal neurons, and patch clamp was used to record suprathreshold and subthreshold activity1 ( Fig . 2a). This study shows that despite intracellular blockade of subthreshold electrical activity with GABA receptor blockers, V1 neurons fire action potentials in a direction-selective manner, suggesting that excitatory input is sufficient to generate direction selectivity.
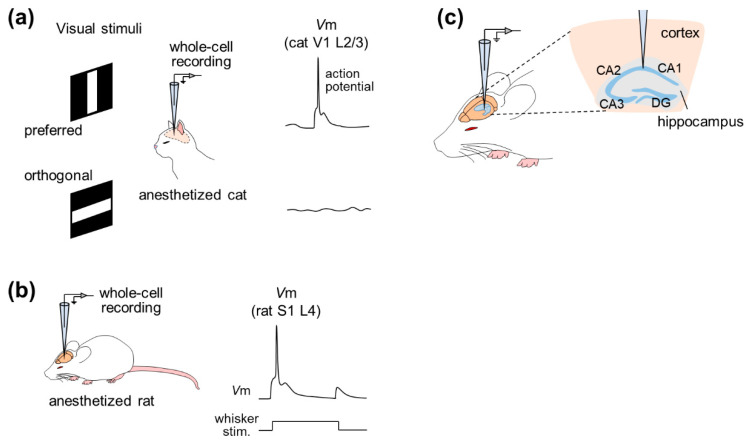
Figure 2 Example of studies based on in vivo whole-cell recordings from anesthetized animals. (a) Whole-cell recordings were performed from neurons in layer 2/3 of the primary visual cortex (V1) of anesthetized cats while the animals were exposed to visual stimuli. In this example, the membrane potential depolarized when the cat saw the preferred (horizontal) stimulus, while the potential remained stable when the animal was exposed to the orthogonal (vertical) stimulus. (b) Whole-cell recordings were performed from neurons in layer 4 of the primary somatosensory cortex (S1) of an anesthetized rat. When rat whiskers were stimulated, the membrane potential of neurons was depolarized. (c) Whole-cell recordings are done from neurons in the hippocampus.
Subsequently, whole-cell recording methods have been widely used, particularly in the rat barrel cortex, a subregion of S1 whose activity correlates with whisker-tactile behavior. Intrinsic properties and subthreshold responses to whisker (specifically tactile whisker) stimulation can be investigated by whole-cell recordings under pentobarbital anesthesia 2 . Then, a series of studies accurately described the dynamic receptive field of whisker deflection through recordings in the rat barrel cortex under polyurethane anesthesia and post hoc reconstruction of recording cells (Fig. 2b). In addition to post hoc visualization or reconstruction, patch-clamp recordings can be combined with genetic manipulation and optical imaging of cortical neurons in urethane-anaesthetized rats 6 . Several studies have used lentiviral vectors for neuron-specific gene delivery, using two-photon microscopy-based techniques to analyze phenotypes at the level of individual cortical cells. This study used high-resolution two-photon time-lapse imaging to monitor the structure of dendritic spines and axons, and simultaneously measured electrophysiological cellular responses through two-photon microscopy-guided whole-cell recordings. This method is well suited for correlating electrophysiological function with gene expression in individual neurons in the intact brain, but its feasibility in awake animals has not been demonstrated.
2.2 Areas such as the hippocampus
In addition to studies of the neocortex, whole-cell recordings from anesthetized animals have also investigated other regions, such as the olfactory cortex, hippocampus, basolateral amygdala, piriform cortex, and thalamus in the brain. There are even studies of the brainstem and cerebellum.
Hahn et al., for the first time, achieved in vivo whole-cell recordings from entorhinal pyramidal cells, hippocampal pyramidal cells, dentate granule cells and even hippocampal interneurons7,8,9 (Fig. 2c). Simultaneous recording of neocortical local field potentials (LFPs) and membrane potentials from CA1 pyramidal cells, CA3 pyramidal cells, and dentate granule cells under urethane anesthesia revealed that these three cell types are regulated by cortical network oscillations, Various functional connections between the neocortex and various subregions of the hippocampus are indicated. Furthermore, the membrane potentials of hippocampal interneurons located at the stratum radiata and lacunar layer-molecular boundaries were almost perfectly synchronized with the state of the neocortex with little delay, suggesting that neocortical activity drives membrane potential fluctuations in hippocampal interneurons. Recently, two-electrode whole-cell recordings have been used to study the relationship between hippocampal neurons 10 .
Whole-cell recordings of neurons in the basolateral amygdala (BLA), located deeper than the hippocampus in vivo. Studies have shown that BLA neurons exhibit slow oscillations that occur at a frequency of about 0.3 Hz. Using somatosensory stimulation (i.e. foot shocks), auditory stimulation, or posterior thalamic stimulation during fluctuating states, studies have shown that oscillatory activity in the BLA is driven by sets of cortical neurons and that these sets control the amygdala in a state-dependent manner Neuronal responses to aversive stimuli; that is, aversive stimuli are effective when the neural network is in a trough state, but ineffective when the neural network is in a peak state11,12,13 .
Brecht and Sakmann in 2002 achieved in vivo whole-cell recordings of thalamic neurons. Because the ventromedial nucleus (VPM) of the thalamus is the primary source of whisker (whisker) drive input in the barrel cortex, they targeted this brain region and described two main classes of VPM neurons: single-whisker firing Cells and polywhiskers excite cells. The former exhibited subthreshold or suprathreshold responses to specific single whisker stimuli, while the latter exhibited responses to multiple whisker stimuli. Furthermore, they demonstrate that the two cell types differ in size of receptive field, pattern of response to whisker deflection, strength and intrinsic properties of inhibitory input 14 .
A series of studies by Häusser’s group explored information processing in the cerebellum. The cerebellum is an advantageous model system for addressing the relationship between sensory-evoked synaptic inputs and the resulting pattern of output spikes, because granule cells in the cerebellum constitute the input layer, translating mossy fiber signals into parallel fiber inputs to the cerebellum. Purkinje cells. For example, Ishikawa et al. Addressed the question of how individual cerebellar granule cells integrate multisensory (ie, somatosensory, auditory, and visual) signals during the input phase of the cerebellar cortex. Using whole-cell voltage-clamp recordings, they describe neuronal responses to sensory, auditory, visual stimuli, or the convergence of these stimuli, and show that a combination of multisensory inputs can enhance granule cell spiking output15,16,17,18,19 .
Blind patch-clamping makes it difficult to specifically locate the recorded cells, so the researchers developed a “targeted patch-clamp” technique to record the membrane potential of specific target cells in the neocortex. There are two methods of “two-photon targeted patch clamp” and “cell shadow patch clamp”.
Margrie et al. Two-photon imaging was first incorporated into an in vivo patch-clamp approach, and an in vivo targeted-clamp technique was developed to guide glass electrodes to individual labeled cortical neurons in vivo (“two-photon targeted-clamp”, Figure 3a) . Margrie et al. used transgenic mice whose parvalbumin-positive interneurons were labeled with enhanced green fluorescent protein (eGFP). Patch-clamp recordings were performed on parvalbumin-positive interneurons in S1. They characterized the intrinsic properties of interneurons, thereby revealing patterns of spontaneous and sensory-evoked activity in S1 neurons 20 . Additionally, Kitamura et al. A shaded patch-clamp method was established in which patch-clamp electrodes were used to perfuse the extracellular space of target neurons with fluorescent dyes, allowing neurons to be visualized as negative images (‘shading’) and identified based on their somatic and dendritic structures. They then placed the same electrodes on neurons under visual control to obtain patch-clamp recordings from visually identified neurons in the neocortex and cerebellum of rats and mice (“shaded patch-clamp”; Figure 3b ). They also utilized targeted in vivo single-cell electroporation of plasmid DNA into identified cell types to achieve stable transgene expression 21 . These techniques accelerate not only electrophysiological recordings, but also labeling and genetic manipulation of individual neurons in intact naive animals.
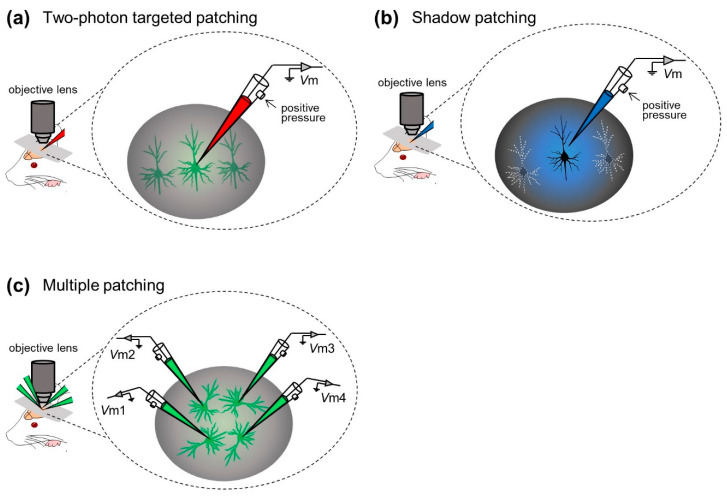
Figure 3. Examples of visual in vivo whole-cell recording techniques. (a) Whole-cell recording of neocortical neurons with the aid of two-photon microscopy. (b) Whole-cell recordings from neocortical neurons under visual monitoring of neuronal morphological shading. (c) Simultaneous whole-cell recordings from up to four neurons.
In most of the aforementioned studies, the researchers captured the membrane potential dynamics of individual neurons in experiments. Especially under “blind insertion” conditions, even single whole-cell recordings can only be achieved probabilistically. In general, simultaneous whole-cell recordings of multiple neurons are technically challenging because the movement of multiple glass electrodes can interfere with each other and disrupt stable seals. However, Jouhanneau et al. This problem was addressed by simultaneous targeted patch-clamp recordings from up to four neocortical neurons (Fig. 3c). They describe similarities or differences in information processing between cells at the subthreshold level and reveal synaptic connections between cortical neurons22,23,24,25 .
3. In vivo whole-cell recording in awake animals
3.1 Neocortex
Margrie et al., started training the rats to remain still in the recording apparatus a few days before surgery and patch-clamp recordings were performed on the rats. This training is laborious but important, as unaccustomed animals will struggle or try to escape the recording device incessantly. The authors first reported whole-cell recordings of rodent barrel cortex in the awake state and observed membrane potential depolarization in response to whisker stimulation 26 . Subsequently, Poulet et al. and Yu et al. demonstrated that the desynchronized state of the mouse whisker barrel cortex during autonomous whiskering behavior was triggered by increased firing activity in the thalamus and correlated with the activity of some interneurons 27,28 .
Various studies have since reported subthreshold responses to external stimuli in conscious rodents and monkeys. In addition, the researchers applied in vivo whole-cell recordings to other cortical regions in awake rodents, including primary motor cortex (M1), anterior lateral motor cortex (ALM), prefrontal cortex, and primary auditory cortex (A1). Notably, Bitzenhofer et al., successfully performed whole-cell recordings in developing neonatal rats 29 . In addition, Guo et al. and Inagaki et al. revealed the membrane potential dynamics of sustained neural activity in the ALM of awake mice performing a learning task 30,31 . More recently, there have been reports of subthreshold membrane potential correlates of social contact in rodents awake 32,33,34 .
3.2 Areas such as the hippocampus
The limbic system is located inside and deeper in the brain than the neocortex. Regarding the use of electrodes for invasive recordings from deep regions in the body, the probability of recording from the intended region itself is low because the exact location of the electrodes in the brain is unpredictable to the experimenter. Furthermore, for successful whole-cell recordings, the electrode tip of the glass electrode must be kept as clean as possible to create a high-resistance seal on the cell membrane. When researchers try to record membrane potentials in deep regions of the body, there is a good chance that some “obstacles” such as extracellular matrix and blood vessels stick to the electrode tips. Dirty tips impede gigaohm sealing, resulting in low success rates for whole-cell recordings from deep regions in both anesthetized and awake animals. Therefore, whole-cell recordings from deep regions in living animals are technically difficult to achieve, but some researchers have attempted to solve this problem. One solution is to remove the neocortex by suction. This article reviews previous studies that have challenged technical issues and provide new insights into the neural correlates of animal behavior.
We focused on the hippocampus and medial entorhinal cortex, both of which are essential for the representation of the external environment. The best-known hippocampal neural correlates of the external environment are place cells, which selectively fire action potentials as animals move through specific locations in the environment (called “place fields”). While this location-specific increase in firing rate is known as the “rate code” of place cells, their precise spike timing relative to the phase of the ongoing hippocampal theta oscillations as animals approach the location field (i.e., “theta phase precession”), called “timecode”. These supra-threshold activities have been described by extracellular recordings, but the intracellular dynamics of position codes remain elusive.
To examine the mechanism of double-codes, Harvey et al. The intracellular dynamics of mouse place cells navigating in a virtual reality environment were first monitored using an in vivo whole-cell recording approach 35 (Fig. 4a). As animals ran back and forth along virtual linear tracks, they acquired robust location-selective firing activity in hippocampal neurons and identified three subthreshold features of location fields: (1) asymmetric ramp-like depolarization of baseline membrane potential , (2) the increase in the amplitude of the intracellular theta oscillations, and (3) the phase precession of the intracellular theta oscillations relative to the extracellularly recorded theta rhythms. These results characterize the intracellular dynamics behind the rate and time codes of place cells. The virtual reality system introduced by Harvey et al. Opens the door to new experimental approaches to study neural circuits for spatial navigation.
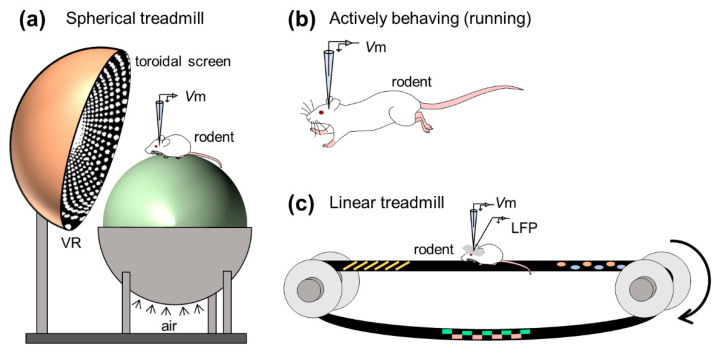
Figure 4 Example of a study of in vivo whole-cell recordings of hippocampal neurons in awake animals. (a) Whole-cell recordings were made from hippocampal neurons of animals running on a spherical treadmill with a virtual reality system. (b) Whole-cell recordings were made from neurons of actively running animals. (c) Simultaneous recording of membrane potential and local field potential from animals running on a linear treadmill.
Whole-cell recordings from freely moving rodents are technically more challenging (Fig. 4b). This is because the rodent’s abrupt behavior during recordings can lead to larger brain movements, which can lead to unstable electrode seals. The high-resistance seal is sensitive to even subtle movements of the brain, as the diameter of the electrode tip is about 3 μm, while the cell bodies of rat and mouse pyramidal neurons are about 20 μm in length. Despite technical difficulties, Lee et al. successfully performed whole-cell recordings in autonomously behaving animals 36 . Epsztein et al. subsequently reported fast events in membrane potentials with amplitudes smaller than the peak potential, termed pre-peak potentials 37 . They then examined intrinsic differences between place cells and quiescent cells (that is, cells that do not fire spikes during environmental exploration) in the CA1 subregion of the hippocampus when rats freely navigate the environment, and found that, compared with quiescent cells, place Cells have a lower spiking threshold. The onset of exploration and future localization cells may exhibit higher burst discharges prior to exploration 38 . In another study, Lee et al. A further attempt was made to artificially induce a spatially homogeneous depolarization of hippocampal cells by injecting a positive current through a glass electrode and found that spatially tuned subthreshold responses and location-specific discharges appeared suddenly and reversibly even in silent cells 39 . Thus, they interpret postsynaptic neuron excitability to gate presynaptic inputs and propose a unique cellular mechanism to generate location codes. Following these studies, the intracellular mechanisms underlying the spatial representation of the rodent limbic system, including the hippocampus and medial entorhinal cortex, have been extensively studied.
The hippocampus generates extracellular electrical oscillations that reflect the ensemble of neural suprathreshold firing and subthreshold synaptic activity and play important roles in learning, memory, and spatial navigation. Characteristic extracellular oscillations (often referred to as LFPs), especially in the hippocampus, are (1) theta oscillations (3-10 Hz) and (2) sharp wave-ripple complexes (SWRs), consisting of sharp waves (2- 30 Hz)) and transient ripple oscillations (100–250 Hz), which contribute to (1) memory encoding and (2) memory consolidation, respectively. To search for membrane potentials associated with learning and memory, researchers routinely capture subthreshold dynamics and field oscillations in the hippocampus of awake rodents, with a particular focus on the SWR, to understand the intracellular characteristics of hippocampal neurons during other frequency bands of extracellular oscillations (Fig. 4c). ).
English DF et al. were the first to use sharp electrodes to achieve intracellular recordings during SWR 40 . They successfully recorded from free-running animals and found consistent large depolarizations in CA1 pyramidal cells during SWR, which are associated with transient ripple frequency fluctuations in membrane potential called intracellular ripples. Note that intracellular ripples are also observed in adjacent regions (ie, the subhippocampus ). A series of subsequent studies precisely characterized subthreshold activity as well as hippocampal SWR. Hulse et al. Whole-cell recordings from animals running on a treadmill revealed that the membrane potential surrounding hippocampal ripple events includes (1) spike-related depolarization, and (2) intracellular high-frequency ripple-like oscillations superimposed on extracellular ripples , and (3) hyperpolarization after corrugation. They further noted that precise activation of individual hippocampal pyramidal cells during SWR requires a balance between excitation and inhibition 42 . These studies of SWR-related intracellular dynamics suggest a synaptic mechanism behind peak discharge output during extracellular ripple events, which would contribute to memory consolidation.
Consistent with its contribution to memory, hippocampus-related diseases are Alzheimer’s disease and epilepsy. For example, Šišková et al. In vivo whole-cell recordings from hippocampal pyramidal neurons in a mouse model of Alzheimer’s disease with simultaneous high-resolution stimulated emission depletion microscopy imaging and computational modeling. They demonstrate that branching structure-dependent amplification of synaptic input to action potential output would represent a cytopathological mechanism of network dysfunction and suggest that this pathological mechanism may be relevant to other neurodegenerative diseases with abnormal dendritic morphology 43 .
So far, we present previous studies investigating synaptic mechanisms underlying synaptic output, particularly focusing on the neocortex, hippocampus, and hippocampal-adjacent regions. In vivo whole-cell recordings in awake mammals have been further achieved in various domains, including the olfactory bulb, thalamus, cerebellum, lateral septum, and inferior colliculus of bats. Note that these in vivo whole-cell recording studies of the inferior colliculus were performed in awake bats, because the inferior colliculus of bats is not covered by the neocortex or cerebellum and can be seen suboptically through the skull.
To be continued
references
- Nelson S., Toth L., Sheth B., Sur M. Orientation selectivity of cortical neurons during intracellular blockade of inhibition. Science. 1994;265:774–777.
- Zhu JJ, Connors BW Intrinsic Firing Patterns and Whisker-Evoked Synaptic Responses of Neurons in the Rat Barrel Cortex. J. Neurophysiol. 1999;81:1171–1183.
- Brecht M., Roth A., Sakmann B. Dynamic Receptive Fields of Reconstructed Pyramidal Cells in Layers 3 and 2 of Rat Somatosensory Barrel Cortex. J. Physiol. 2003;553:243–265.
- Manns ID, Sakmann B., Brecht M. Sub- and suprathreshold receptive field properties of pyramidal neurones in layers 5A and 5B of rat somatosensory barrel cortex. J. Physiol. 2004;556:601–622.
- Brecht M., Sakmann B. Dynamic representation of whisker deflection by synaptic potentials in spiny stellate and pyramidal cells in the barrels and septa of layer 4 rat somatosensory cortex. J. Physiol. 2002;543:49–70.
- Dittgen T., Nimmerjahn A., Komai S., Licznerski P., Waters J., Margrie TW, Helmchen F., Denk W., Brecht M., Osten P. Lentivirus-based genetic manipulations of cortical neurons and their optical and Electrophysiological monitoring in vivo. Proc. Natl. Acad. Sci. USA. 2004;101:18206–18211.
- Hahn TTG, McFarland JM, Berberich S., Sakmann B., Mehta MR Spontaneous persistent activity in entorhinal cortex modulates cortico-hippocampal interaction in vivo. Nat. Neurosci. 2012;15:1531–1538. doi: 10.1038/nn.3236.
- Hahn TTG, Sakmann B., Mehta MR Differential responses of hippocampal subfields to cortical up-down states. Proc. Natl. Acad. Sci. USA. 2007;104:5169–5174. doi: 10.1073/pnas.0700222104.
- Hahn TTG, Sakmann B., Mehta MR Phase-locking of hippocampal interneurons’ membrane potential to neocortical up-down states. Nat. Neurosci. 2006;9:1359–1361. doi: 10.1038/nn1788.
- Liu Y.-Z., Wang Y., Shen W., Wang Z. Enhancement of synchronized activity between hippocampal CA1 neurons during initial storage of associative fear memory. J. Physiol. 2017;595:5327–5340. doi: 10.1113/ JP274212.
- Windels F., Yan S., Stratton PG, Sullivan R., Crane JW, Sah P. Auditory Tones and Foot-Shock Recapitulate Spontaneous Sub-Threshold Activity in Basolateral Amygdala Principal Neurons and Interneurons. PLoS ONE. 2016;11:e0155192. doi: 10.1371/journal.pone.0155192.
- Windels F., Crane JW, Sah P. Inhibition Dominates the Early Phase of Up-States in the Basolateral Amygdala. J. Neurophysiol. 2010;104:3433–3438. doi: 10.1152/jn.00531.2010.
- Crane JW, Windels F., Sah P. Oscillations in the basolateral amygdala: Aversive stimulation is state dependent and resets the oscillatory phase. J. Neurophysiol. 2009;102:1379–1387. doi: 10.1152/jn.00438.2009.
- Brecht M., Sakmann B. Whisker maps of neuronal subclasses of the rat ventral posterior medial thalamus, identified by whole-cell voltage recording and morphological reconstruction. J. Physiol. 2002;538:495–515. doi: 10.1113/jphysiol.2001.012334 .
- Chadderton P., Margrie TW, Häusser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–860. doi: 10.1038/nature02442.
- Rancz EA, Ishikawa T., Duguid I., Chadderton P., Mahon S., Häusser M. High-fidelity transmission of sensory information by single cerebellar mossy fibre boutons. Nature. 2007;450:1245–1248. doi: 10.1038/ nature05995.
- Duguid I., Branco T., London M., Chadderton P., Häusser M. Tonic Inhibition Enhances Fidelity of Sensory Information Transmission in the Cerebellar Cortex. J. Neurosci. 2012;32:11132–11143. doi: 10.1523/JNEUROSCI. 0460-12.2012.
- Ishikawa T., Shimuta M., Häusser M. Multimodal sensory integration in single cerebellar granule cells in vivo. Elife. 2015;4:e12916. doi: 10.7554/eLife.12916.
- Duguid I., Branco T., Chadderton P., Arlt C., Powell K., Häusser M. Control of cerebellar granule cell output by sensory-evoked Golgi cell inhibition. Proc. Natl. Acad. Sci. USA. 2015;112 :13099–13104. doi: 10.1073/pnas.1510249112.
- Margrie TW, Meyer AH, Caputi A., Monyer H., Hasan MT, Schaefer AT, Denk W., Brecht M. Targeted Whole-Cell Recordings in the Mammalian Brain In Vivo. Neuron. 2003;39:911–918. doi : 10.1016/j.neuron.2003.08.012.
- Kitamura K., Judkewitz B., Kano M., Denk W., Häusser M. Targeted patch-clamp recordings and single-cell electroporation of unlabeled neurons in vivo. Nat. Methods. 2008;5:61–67. doi: 10.1038 /nmeth1150.
- Jouhanneau J.-S., Ferrarese L., Estebanez L., Audette NJ, Brecht M., Barth AL, Poulet JFA Cortical fosGFP Expression Reveals Broad Receptive Field Excitatory Neurons Targeted by POm. Neuron. 2014;84:1065–1078. doi: 10.1016/j.neuron.2014.10.014.
- Jouhanneau J.-S., Kremkow J., Poulet JFA Single synaptic inputs drive high-precision action potentials in parvalbumin expressing GABA-ergic cortical neurons in vivo. Nat. Commun. 2018;9:1540. doi: 10.1038/s41467-018 -03995-2.
- Jouhanneau J.-S., Poulet JFA Multiple Two-Photon Targeted Whole-Cell Patch-Clamp Recordings From Monosynaptically Connected Neurons in vivo. Front. Synaptic Neurosci. 2019;11:15. doi: 10.3389/fnsyn.2019.00015.
- Jouhanneau J.-S., Kremkow J., Dorrn AL, Poulet JFA In Vivo Monosynaptic Excitatory Transmission between Layer 2 Cortical Pyramidal Neurons. Cell Rep. 2015;13:2098–2106. doi: 10.1016/j.celrep.2015.11.011 .
- Margrie TW, Brecht M., Sakmann B. In vivo, low-resistance, whole-cell recordings from neurons in the anaesthetized and awake mammalian brain. Pflügers Arch. 2002;444:491–498. doi: 10.1007/s00424-002- 0831-z.
- Poulet JFA, Fernandez LMJ, Crochet S., Petersen CCH Thalamic control of cortical states. Nat. Neurosci. 2012;15:370–372. doi: 10.1038/nn.3035.
- Yu J., Hu H., Agmon A., Svoboda K. Recruitment of GABAergic Interneurons in the Barrel Cortex during Active Tactile Behavior. Neuron. 2019;104:412–427.e4. doi: 10.1016/j.neuron.2019.07. 027.
- Bitzenhofer SH, Sieben K., Siebert KD, Spehr M., Hanganu-Opatz IL Oscillatory Activity in Developing Prefrontal Networks Results from Theta-Gamma-Modulated Synaptic Inputs. Cell Rep. 2015;11:486–497. doi: 10.1016/j .celrep.2015.03.031.
- Guo ZV, Inagaki HK, Daie K., Druckmann S., Gerfen CR, Svoboda K. Maintenance of persistent activity in a frontal thalamocortical loop. Nature. 2017;545:181–186. doi: 10.1038/nature22324.
- Inagaki HK, Fontolan L., Romani S., Svoboda K. Discrete attractor dynamics underlies persistent activity in the frontal cortex. Nature. 2019;566:212–217. doi: 10.1038/s41586-019-0919-7.
- Lenschow C., Brecht M. Barrel Cortex Membrane Potential Dynamics in Social Touch. Neuron. 2015;85:718–725. doi: 10.1016/j.neuron.2014.12.059.
- Ebbesen CL, Doron G., Lenschow C., Brecht M. Vibrissa motor cortex activity suppresses contralateral whisking behavior. Nat. Neurosci. 2017;20:82–89. doi: 10.1038/nn.4437.
- Clemens AM, Lenschow C., Beed P., Li L., Sammons R., Naumann RK, Wang H., Schmitz D., Brecht M. Estrus-Cycle Regulation of Cortical Inhibition. Curr. Biol. 2019;29:605 –615.e6. doi: 10.1016/j.cub.2019.01.045.
- Harvey CD, Collman F., Dombeck DA, Tank DW Intracellular dynamics of hippocampal place cells during virtual navigation. Nature. 2009;461:941–946. doi: 10.1038/nature08499.
- Lee AK, Manns ID, Sakmann B., Brecht M. Whole-Cell Recordings in Freely Moving Rats. Neuron. 2006;51:399–407. doi: 10.1016/j.neuron.2006.07.004.
- Epsztein J., Lee AK, Chorev E., Brecht M. Impact of Spikelets on Hippocampal CA1 Pyramidal Cell Activity During Spatial Exploration. Science. 2010;327:474–477. doi: 10.1126/science.1182773.
- Epsztein J., Brecht M., Lee AK Intracellular Determinants of Hippocampal CA1 Place and Silent Cell Activity in a Novel Environment. Neuron. 2011;70:109–120. doi: 10.1016/j.neuron.2011.03.006.
- Lee D., Lin B.-J., Lee AK Hippocampal Place Fields Emerge upon Single-Cell Manipulation of Excitability During Behavior. Science. 2012;337:849–853. doi: 10.1126/science.1221489.
- English DF, Peyrache A., Stark E., Roux L., Vallentin D., Long MA, Buzsáki G. Excitation and compete inhibition to control spiking during hippocampal ripples: Intracellular study in behaving mice. J. Neurosci. 2014;34: 16509–16517. doi: 10.1523/JNEUROSCI.2600-14.2014.
- Böhm C., Peng Y., Maier N., Winterer J., Poulet JFA, Geiger JRP, Schmitz D. Functional Diversity of Subicular Principal Cells during Hippocampal Ripples. J. Neurosci. 2015;35:13608–13618. doi: 10.1523 /JNEUROSCI.5034-14.2015.
- Hulse BK, Moreaux LC, Lubenov EV, Siapas AG Membrane Potential Dynamics of CA1 Pyramidal Neurons during Hippocampal Ripples in Awake Mice. Neuron. 2016;89:800–813. doi: 10.1016/j.neuron.2016.01.014.
- Šišková Z., Justus D., Kaneko H., Friedrichs D., Henneberg N., Beutel T., Pitsch J., Schoch S., Becker A., von der Kammer H., et al. Dendritic Structural Degeneration Is Functionally Linked to Cellular Hyperexcitability in a Mouse Model of Alzheimer’s Disease. Neuron. 2014;84:1023–1033. doi: 10.1016/j.neuron.2014.10.024.
This article is reprinted from: https://www.insidentally.com/articles/000024/
This site is for inclusion only, and the copyright belongs to the original author.