Author | Wan Chen
Editor | Jing Yu
Imagine if you are a heart patient, the “engine” that supports your life is about to shut down, and replacing someone else’s heart is the only option. However, the doctor regrets to tell you that even if you are on the shortlist, you still need 3 years to wait for a transplantable heart. How would you feel?
In fact, hundreds of thousands of patients are caught in such an unsolved predicament every year. According to data, in China, only 6% of patients who can be matched to donated organs in a timely manner. Looking at the world, this number cannot be raised higher – after all, the number of organs that can be donated is extremely limited.
In order to expand the “supply” of donated organs, scientists have even thought of a plan that sounds a bit ridiculous: transplanting animal organs into humans. For many people, such an idea is unheard of or even appalling. In fact, this possibility has been explored for decades, although it is no easier than landing on the moon.
However, the good news has arrived.
On January 7, 2022, 57-year-old Bannet David received a pig heart transplant because of heart failure and was not suitable for other treatment methods, and extended his life for nearly two months (59 days). In this regard, industry insiders said that their expectations were exceeded; after all, David also suffered from serious underlying diseases and complications before the operation.

On May 9, 2022, the University of Maryland School of Medicine honors David Bennet, the first in history to receive a transgenic pig heart transplant
In the previous half a year, 3 brain-dead patients received pig kidney transplants one after another, and during the observation period, the pig kidney function was working well.
In the last month, two more brain-dead patients also received pig heart transplants, and no hyperacute immune rejection occurred during the 72-hour observation period.
Together, these six cases send a signal: pigs are bringing hope to the shortage of human organs .
For researchers and entrepreneurs who have been in the dark for decades, such hope is a shot in the arm. If the technical route of pig as a human organ donor is verified and feasible, it will be as significant as the overcoming of cancer, and it will become the Holy Grail of the medical profession, extending the lives of hundreds of thousands of people every year.
As the protagonist of transplanted organs, “donor pigs” are also taking the lead.
The “research and development” cost of each of these seemingly ordinary pigs is about one million yuan, which is “too expensive.” Likewise, companies that develop and breed donor pigs are starting to receive more attention, which seems to be pushing pigs forward as human organ donors.
“Paige” takes the lead
In fact, the technical route of transplanting organs from animals to humans, that is, xenotransplantation, is the originator of organ transplantation.
Hundreds of years ago, the first organ donors that humans thought of were animals. At that time, there were no organ donations, and no human organs could be used for transplantation. However, due to the ineffectiveness of transplanting animal organs into humans, human donation came to mind. After years of exploration and experimentation, at present, the donor’s organs have become the most effective means of organ transplantation.
The new difficulty, however, is that the number of human organs available has always been very limited and in short supply. Xenogeneic organs “have to” come into people’s sight again.
But this time around, xenotransplantation has been explored for nearly a century. Professor Chen Zhonghua, who has been deeply involved in the field for 40 years, summed up this exploration process as “four ice-breaking journeys”, and the “freezing periods” experienced in the middle were 37 years, 11 years, and 30 years respectively. He said that every time it encountered bottlenecks and failures, the exploration was forced to suspend until the next opportunity.
Apparently, xenotransplantation had been sitting on the bench for 30 years before the most recent “glaciers melted.” Professor Chen Zhonghua called it “30 years of silence”, but it was not 30 years of silence.
In the 1990s, pigs were regarded as the ideal organ donors instead of monkeys. This is because pigs have organs similar in size to humans, are easy to genetically modify, and have the advantages of rapid reproduction. Gene-edited pigs can minimize immune rejection of human organs. Xenotransplantation has entered the research and exploration stage of “pig as a donor”. At the same time, the development of biotechnology such as cloning and immunosuppressive agents has once again provided impetus for the ice-breaking of xenotransplantation.
The baht accumulated, until the story that happened in the last year finally broke the 30-year silence.
In September, November, and December 2021, two American teams transplanted gene-edited pig kidneys to three brain-dead human recipients, and the transplanted organs survived for more than 2 days without hyperacute immune rejection.
These three cases are surprising because they are hard to come by.
Previously, gene-edited pig organs could only be transplanted into non-human primates (such as monkeys) to verify their safety and efficacy, and were not allowed to be clinically tested in humans. But while nonhuman primates are 80 to 90 percent genetically similar to humans, they are still very different. This forms a paradox: even if pig organs are continuously transplanted into monkeys, they cannot replace humans, and if the safety and efficacy of transplantation into humans cannot be verified, they cannot be used in the clinic.
The paradox didn’t take a turn until NYU professor Robert Montgometry suggested it. Professor Montgometry believes that a subclinical model can be inserted between monkeys and humans, that is, brain-dead patients. If the experiment is successful, the controversy of entering human experiments will not be so big. This clever proposal was finally approved by the U.S. Food and Drug Administration (FDA) in 2019.
The most exciting thing happened on January 7, 2022, a patient with severe heart disease, David Bennett, was not suitable for a human heart transplant. Attending physician Bartley Griffith applied to the FDA for special approval for compassionate use, which allows doctors to resort to experimental treatments after exhausting all treatment options. David himself agreed to a transplant of a gene-edited pig heart.
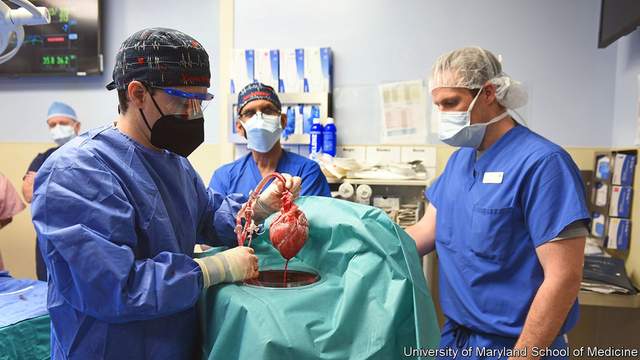
David’s gene-edited pig heart | Source: University of Maryland School of Medicine
After the surgery, David was in good health and watched the Super Bowl accompanied by paramedics. In the end, the gene-edited pig heart lasted 59 days for David.
So far, the gene-edited pig, which has been explored for decades, has finally been verified in humans for the first time by “accidental”, and it has lasted nearly two months of human life.
This “first time” is exciting. In contrast, the first human-to-human heart transplant in 1967 only allowed the patient to survive for 18 days; the xenotransplantation in 1984, in which a little girl named Baby Fae was transplanted The baboon’s heart also survived for only 21 days.
There is no doubt that David’s case is a milestone in xenotransplantation.
Previously, due to ethical regulations, gene editing pigs have been difficult to verify by humans, which is a more challenging part of this innovation track. So when Bannet David accepted the pig’s heart by chance and survived for two months, the case was extremely cherished by the industry and outside, and David’s interpretation of the cause of death has also become a standard to verify whether the current technical route is mature or even right or wrong . It has “high hopes” that this will be the dawn before the dawn of pig success as a xenogeneic organ donor.
After all, this is the biggest breakthrough in two decades. If this technological route is successful, it will bring hope of survival to hundreds of thousands of people.
Finally, on June 22, 2022, the “inspection report” came.
The New England Journal of Medicine, one of the four top medical journals, published a paper on Bannet David’s acceptance of gene-edited pig hearts, and the cause of death was disclosed in detail by the team of attending physicians for the first time.
The results show that the current technology of xenotransplantation can solve the problem of hyperacute immune rejection between pigs and humans, but the cause of David’s heart failure remains to be further explored, which may be the infection of porcine giant cells or other unknown reasons. Impact. That is to say, although the prospects of this technical route are promising, more clinical cases are needed to test and iterate experience. It is worth noting that the US FDA will judge the possibility of clinical trials based on this in the near future .
When David’s case brought new hope for xenotransplantation, piglets as organ donors, and their R&D and breeding companies, also became the focus of attention.
How to create a “Million Golden Pig”
It is conceivable that to “bring” a good donor pig that can be used for organ transplantation is completely different from the meat pigs that are generally used for food. Pan Dengke, the founder of Zhongke Auger, which focuses on the development and cultivation of organ donor pigs, told Geek Park that these piglets are “so expensive that no one can afford to talk.”
Unlike the meat pigs that are sold after six or seven months of raising, the donor pigs bred by Zhongke Oger are small pigs, whose organs are similar in size to those of the Chinese and well-proportioned. For the sake of stable genetics and batch breeding, such pigs are also required to have better disease resistance and reproduction. In this way, the gene-edited donor pigs are bred and iterated from generation to generation, and they may eventually become “humanized” pigs. Put such pig organs into the human body, and the human body can recognize “it is “our own”. , then accept it.
Since the organs of the donor pigs will be used to save lives in the future, the requirements for the environment in which they are maintained—the breeding bases are quite strict. The staff said that it is to “keep it like a baby, even if the hotel (guests) or ICU patients can’t live in such a high-level place.”
This is especially reflected in the related initiatives of biosecurity. The breeding base of donor pigs is required to meet the environmental standards of “ultra-clean basis”, and the less pathogenic microorganisms are carried, the better. Under the care of breeders who specialize in animal experiments, all the milk eaten by the donor pigs must be sterilized, and the feed formula must also be traced to prevent any potential microbial infection.
Interestingly, donor pigs also need to undergo caesarean section like humans when giving birth. This is not to fear that it will be difficult to give birth, but to prevent piglets from passing through the birth canal (there may be microorganisms), and wrap them in a relatively sterile uterus. The piglets are cut out and placed in a sterilized incubator for careful maintenance.
From the moment the piglet is born, files tracking its entire life cycle are recorded and monitored 24 hours a day, 365 days a year. In this way, what is its pedigree, genetic identification records, what diseases, what conditions, and even daily physiological and biochemical indicators are “traceable”.
After that, when the pig of what genotype is needed for breeding and breeding, the pig “goes” to test the effect, one generation, two generations, three generations… Iterative optimization is continued, and as a product, it is continuously improved until it reaches standard for clinical use. At present, the GTKO miniature pig of Zhongke Auger has been iterated for 11 generations.

Organ Donor——GTKO Wuzhishan Miniature Pig|Photo Source: Zhongke Auger
In fact, there is a consensus in the industry on which major genes to knock out or insert, namely: knocking out several specific pig genes to prevent immune rejection and abnormal organ growth; at the same time, knocking in several specific human transgenes , to promote the expression of regulatory functional proteins.
So why do you need to keep exploring gene combinations and breeding iterations? Even iterative genotypes have become the core competitiveness of breeding donor pigs.
Pandenke explained: ” Getting a very good humanized genetic pig is like winning the lottery .” “It is easy to knock out or insert which genes, but it is not so easy to express a gene, and after verifying the effect, these genes It can be inherited stably, and others have consistent effects.” This requires a lot of cross-disciplinary key technologies and clinical experience.
The technical accumulation of Zhongke Auger is inseparable from the experience of the founder Pan Dengke himself. In 2005, Pan Dengke graduated from China Agricultural University with a Ph.D. In this year, he and his team made the first somatic cell cloned pig in China. Since 2008, he has focused on the research of xenotransplantation, and in 2018, he decided to build an industrialized platform for xenotransplantation and founded Zhongke Auger.
An example is, “An American colleague edited a gene and the expression effect is very good, but you may not be able to imitate it after six or seven years.” If the expression effect of a gene is not good, then try to replace other genes. After modifying one or two genes, it is necessary to keep breeding and breeding until the gene combination with the most ideal expression effect appears, and then a stable product can be cultivated and a germline can be cultivated.
This is also the ultimate goal of breeding donor pigs. On the one hand, it is necessary to iterate the pigs that can truly meet the standards of the Master. After the human body receives gene-edited pig organ transplantation, the effect of the transplant is at least the same as or even better than the transplantation of human organ donation. On the other hand, in order to achieve consistent mass production of donor pigs and achieve a stable level of production, we can respond to market demands.
Donor pig, take off to the wind
In theory, once the genotypes of the pigs are determined, the organs can be factory-standardized and “produced” like the production of industrial products. This means that patients can be transplanted at any time and treated as needed, regardless of timing, quantity and quality.
Based on this prospect, some companies have begun to cultivate such donor pigs industrially. From gene editing, to cloning and breeding, to germline breeding, they have gradually established a one-stop breeding platform for donor pigs.
As early as 2004, the German team invested a lot of money in the industrial layout for the next 20 years, cultivating genetically modified pigs and xenogeneic islets, hearts and kidneys.
In the United States, the exploration of donor pigs is more active, and many cases of industry-university research and development have emerged.
This time it was industry leader Revivicor who supplied the pig hearts to Bannet David. Revivicor’s predecessor, PPL Therapeutics, successfully made the world’s first cloned sheep, Dolly, in 1996, with profound technical accumulation. Notably, in 2011, Revivicor was acquired by the US biopharmaceutical giant United Therapeutics.
As far as exploration in the field of xenotransplantation is concerned, in 2001, Revivicor successfully bred alpha-gal knockout pigs. In 2020, the pig received FDA approval to be used as an alternative for human therapy.
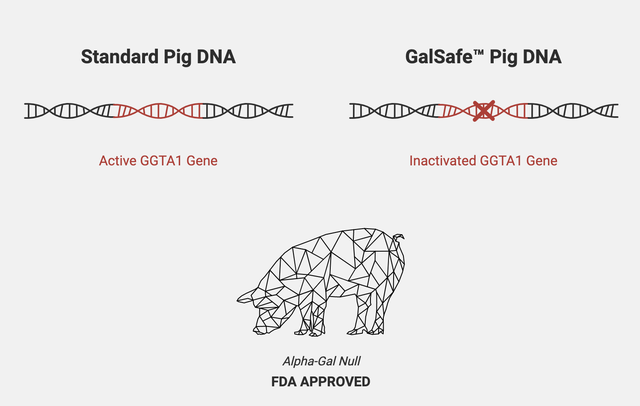
Revivicor’s Galsafe pig knocks out the pig gene for immune rejection in humans | Source: Revivicor official website
Another company, eGenesis, founded in 2015, has broken through the xenotransplantation program in the direction of gene editing. Its founders, Professor George Church and Dr. Yang Luhan, are from Harvard University. The team hopes to use the CRISPR gene editing tool to create safe and human-compatible pig organs. In 2019, eGenesis raised $125 million in Series C financing and plans to file for clinical trials by the end of 2022.
In addition, there are also some Chinese companies in this industry. Among them, Hunan Sino, a well-known company in the industry, has been deeply involved in this field for 20 years; their ultimate goal is to relieve the plight of diabetic patients through xenotransplantation of porcine islets.
Obviously, entrepreneurs in this track need to cultivate for a long time, and the entrepreneurs who are in it need a strong belief to persevere. After all, for the technical route of donor pigs, there is no certain future in sight.
And such beliefs often come from special empathy. On the one hand, entrepreneurs who have plunged into this track have seen the technical prospects of donor pigs—relieving countless life plights; on the other hand, many people bet on this track for reasons that cannot be rejected: they were once patients, or There are such patients in the family.
The aforementioned Professor Robert Montgometry, who proposed the subclinical model, was a heart patient. Four years ago, he underwent a heart transplant, which prompted him to think about xenotransplantation options. In recent years, he has been committed to promoting xenotransplantation: from proposing subclinical models, breaking the paradox that cannot be clinically implemented, to finally practicing subclinical models (the two cases of pig kidney transplantation mentioned above in brain-dead patients were performed by Montgometry team-led), and he continues to push donor pigs into the clinic.
Indeed, other than for reasons like this, it is hard to imagine the possibility of burning money for decades just to change a life. After all, the overall technical route of xenotransplantation is still at an early stage, with great uncertainty and a long blood return cycle. Especially in the past ten years, high investment, long cycle, and high uncertainty are projects that investors are reluctant to touch.
Fortunately, this predicament seems to be loosening.
In the past year, the clinical trials of pig organ masters have made more people see the prospect of donor pigs, and more people have begun to understand the technical route to solve the organ shortage. This keeps the entrepreneurs involved in overall optimism. After all, innovation breakthroughs in many fields are driven by extreme cases.
At the same time, under the concept of hard technology, many investors have begun to turn their attention to the biomedical track, so xenotransplantation has more possibilities to be seen.
Of course, this track still needs more experience to accumulate, but more people participating in this event have a greater chance of winning.
Just like the vision of Thomas Starzl, the father of modern transplantation who has gone through 3 cycles of xenotransplantation: History tells us that what was unthinkable yesterday and barely achievable today will often become routine tomorrow.
This article is reproduced from: https://www.geekpark.net/news/305171
This site is for inclusion only, and the copyright belongs to the original author.