Original link: https://kaopubear.top/blog/2022-08-08-2022q2-ngs-precision-oncology-revenue/
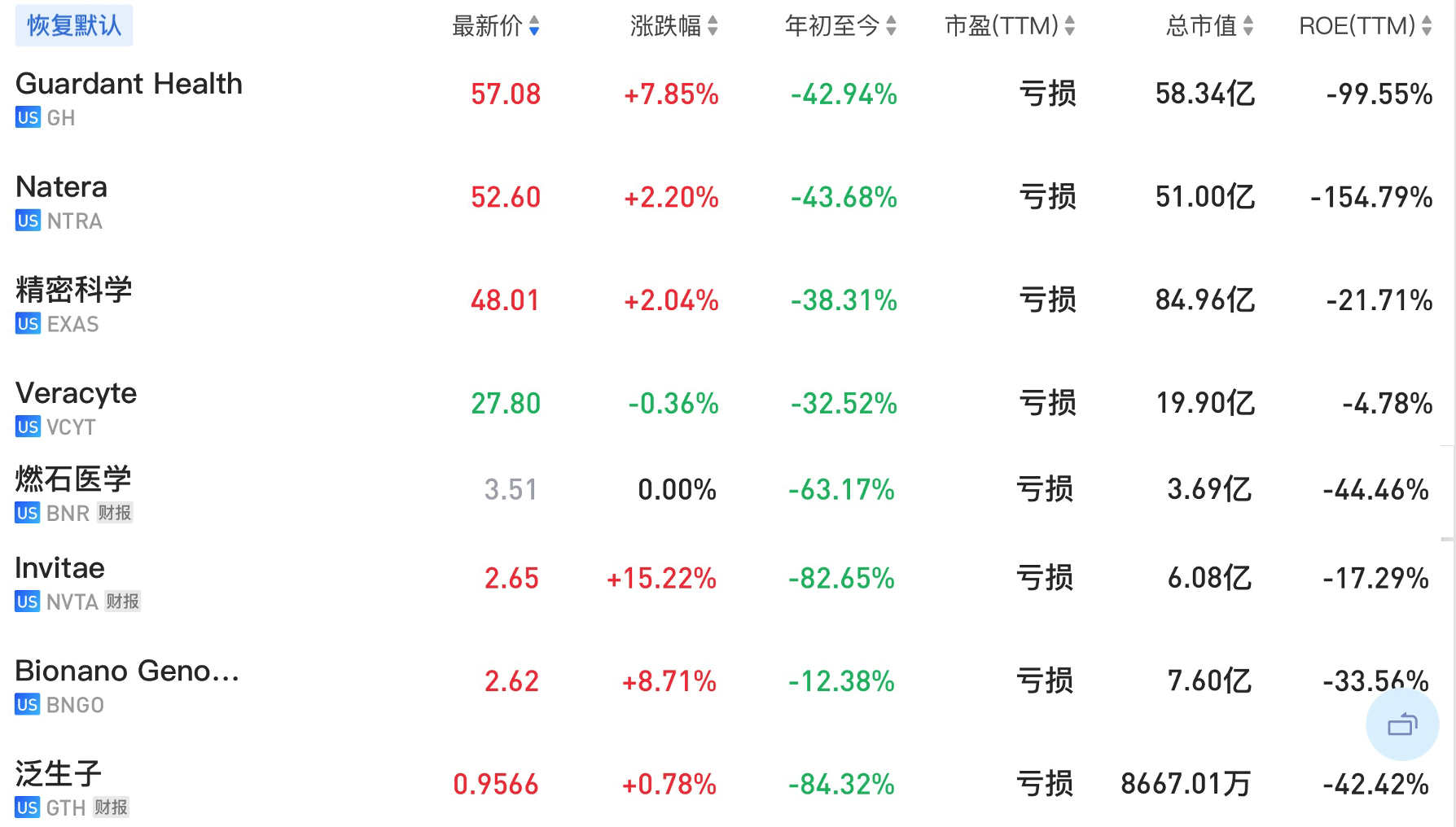
5 U.S.-listed companies are temporarily included, namely Myriad, Natera, Guardant, Veracyte and Exact Sciences. Invitae will be released on August 9. The two domestic companies listed on the U.S. stock market, Burning Rock Medicine and Genetron Health, will be released later. be added together.
The stock prices of these companies on August 8, 2022 are shown in the chart below.
Myriad
News Release Detail | Myriad Genetics
Highlights :
- Revenue of $179.3 million for the quarter ended June 30, 2022
- Excluding revenue from divested businesses, revenue increased 7% year-over-year and 9% sequentially from the first quarter of 2022
- Diluted GAAP earnings per share (EPS) of $ (0.18) and adjusted EPS of $0.04 in the second quarter of 2022
- Fiscal year 2022 financial guidance updated to reflect additional $20 million investment in research and development, technology, and sales and marketing programs
- Ended the quarter with $283.6 million in cash, cash equivalents and investments
Oncology
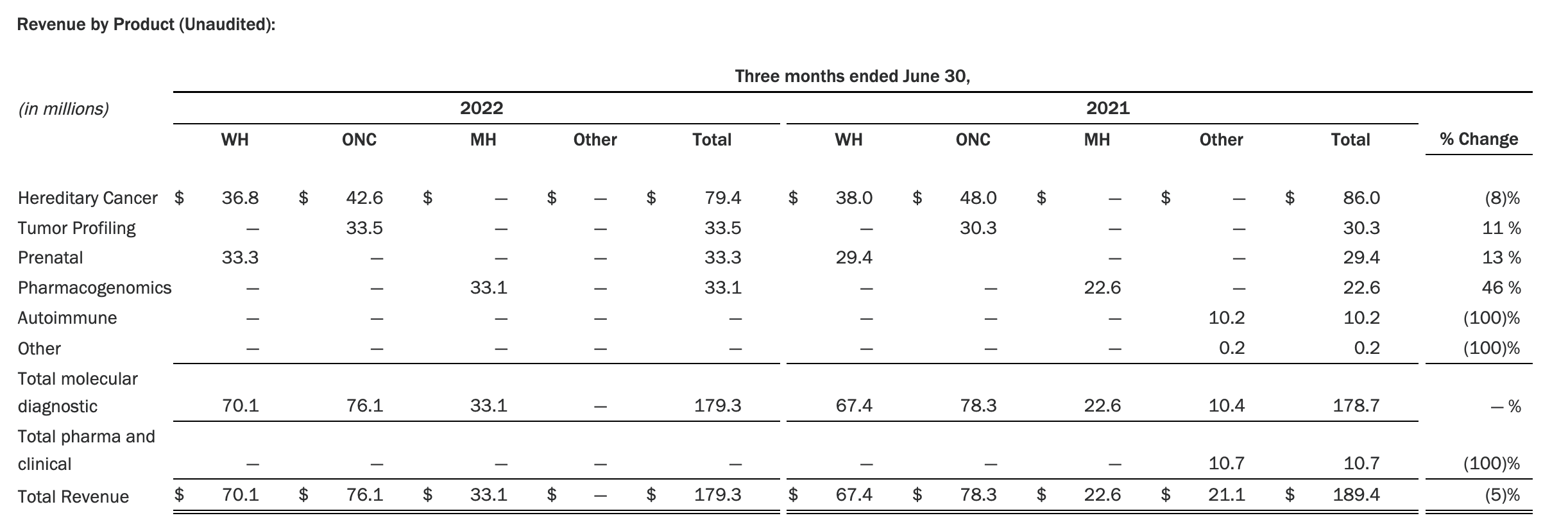
The Myriad Genetics Oncology business provides hereditary cancer testing, including the MyRisk™ hereditary cancer test for patients who have cancer. It also provides tumor profiling products such as the myChoice® CDx companion diagnostic test, the Prolaris® prostate cancer test, and the EndoPredict® breast cancer prognostic test. The Oncology business delivered revenue of $76.1 million in the second quarter of 2022, a decrease of 3% year-over-year and an increase of 9% sequentially from the first quarter of 2022.
- Myriad Genetics recently launched Precise Oncology Solutions, which combines the company’s MyRisk germline cancer testing technology and its myChoice CDx companion diagnostic test with Precise Tumor, a tumor profiling test powered by Illumina, Inc.’s TruSight™ Oncology 500 (TSO500) assay and processed by Intermountain Precision Genomics.
- MyChoice CDx reported its highest quarterly volume level in the United States ever in the second quarter of 2022 with quarterly volumes up 63% year-over-year and 10% sequentially from the first quarter of 2022.
- Prolaris is a prostate cancer prognostic test designed to assess prostate cancer aggressiveness. The Oncology business achieved its highest quarterly Prolaris volumes in the second quarter of 2022, beating its previous quarterly volume record by 6%.
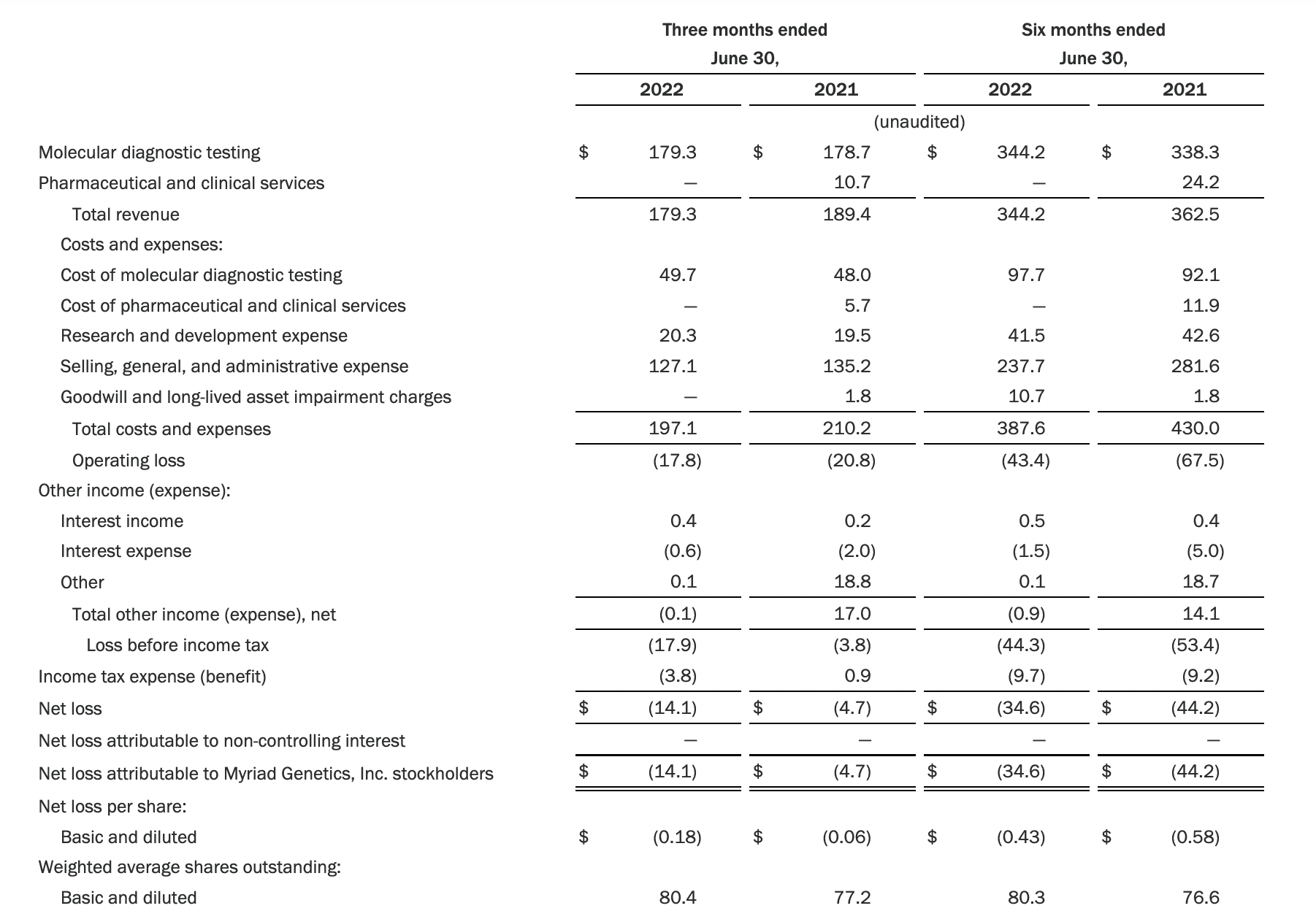
Myriad’s Q2 2022 revenue fell 5% year over year. Total revenue for the period was $179.3 million, down from $189.4 million in the prior year’s second quarter. Myriad posted a net loss of $14.1 million in the quarter, compared with a loss of $4.7 million a year earlier. Ended the quarter with $105.2 million in cash and cash equivalents.

Natera
Natera Reports Second Quarter 2022 Financial Results | Natera
- Generated total revenues of $198.2 million in the second quarter of 2022 compared to $142.0 million in the second quarter of 2021, an increase of 39.6%. Product revenues grew 39.3% over the same period.
- Processed approximately 499,900 tests in the second quarter of 2022, compared to approximately 375,700 tests processed in second quarter of 2021, an increase of 33.0%.
- 2022 revenue guidance raised to $805 million – $825 million.
- Selected to participate in UnitedHealthcare’s Preferred Laboratory Network after a rigorous review process.
- Publication of the Trifecta study for Prospera Kidney in Transplantation; largest prospective, multi-site, fully biopsy matched study to date.
- Completed enrollment in RenaCARE study for Renasight, with more than 1,700 patients across 30+ sites.
- Secured Medicare coverage for muscle invasive bladder cancer; fourth coverage decision for Signatera.
- Presented substantial new Signatera data sets at the 2022 ASCO Annual Meeting.
- Appointed Dr. Minetta Liu as CMO for Oncology.
- Additional equity investment in Natera by Executive Chairman Matt Rabinowitz.
Total revenue rose 40% in the second quarter, driven by strong growth at Signatera, particularly in colorectal and immunotherapies. Revenue increased to $198.2 million, compared with $142 million a year earlier.
Product revenue for the second quarter of 2022 rose 39% to $194.6 million, compared to $139.6 million in the year-ago quarter. Progress so far this year gives the company the confidence to achieve cash flow balance by mid-2024.
Natera posted a net loss of $145.2 million in the second quarter, compared with a net loss of $116 million in the same period in 2021.
As of June 30, 2022, Natera had approximately $638.7 million in cash, cash equivalents, short-term investments and restricted cash (of which $91 million in cash and cash equivalents) compared to $914.5 million in cash as of December 31, 2021 USD (of which 84 million in cash and cash equivalents).
Guardant
Guardant Health, Inc. – Guardant Health Reports Second Quarter 2022 Financial Results
Recent Highlights
- Revenue of $109.1 million for the second quarter of 2022, an increase of 19% over the corresponding period of 2021
- Reported 29,300 tests to clinical customers and 6,000 tests to biopharmaceutical customers in the second quarter of 2022, representing an increase of 40% and 65%, respectively, over the second quarter of 2021
- Received Medicare Coverage for Guardant Reveal™, the first blood-only liquid biopsy test for molecular residual disease testing now covered for certain fee-for-service Medicare patients in the US with stage II or III colorectal cancer
- Completed the purchase of the Guardant Health AMEA Joint Venture, creating a unified organization to expand commercialization of Guardant Health’s industry-leading liquid biopsy technology across the region
- Executed a strategic partnership agreement to offer comprehensive genomic profiling tests to biopharmaceutical companies in China with Adicon, a leading independent clinical laboratory company
- The Company’s Shield LDT launch was well received by clinicians and patients and with early activity exceeding expectations
- Expect both ECLIPSE readout and PMA submission for Shield assay during the second half of the year
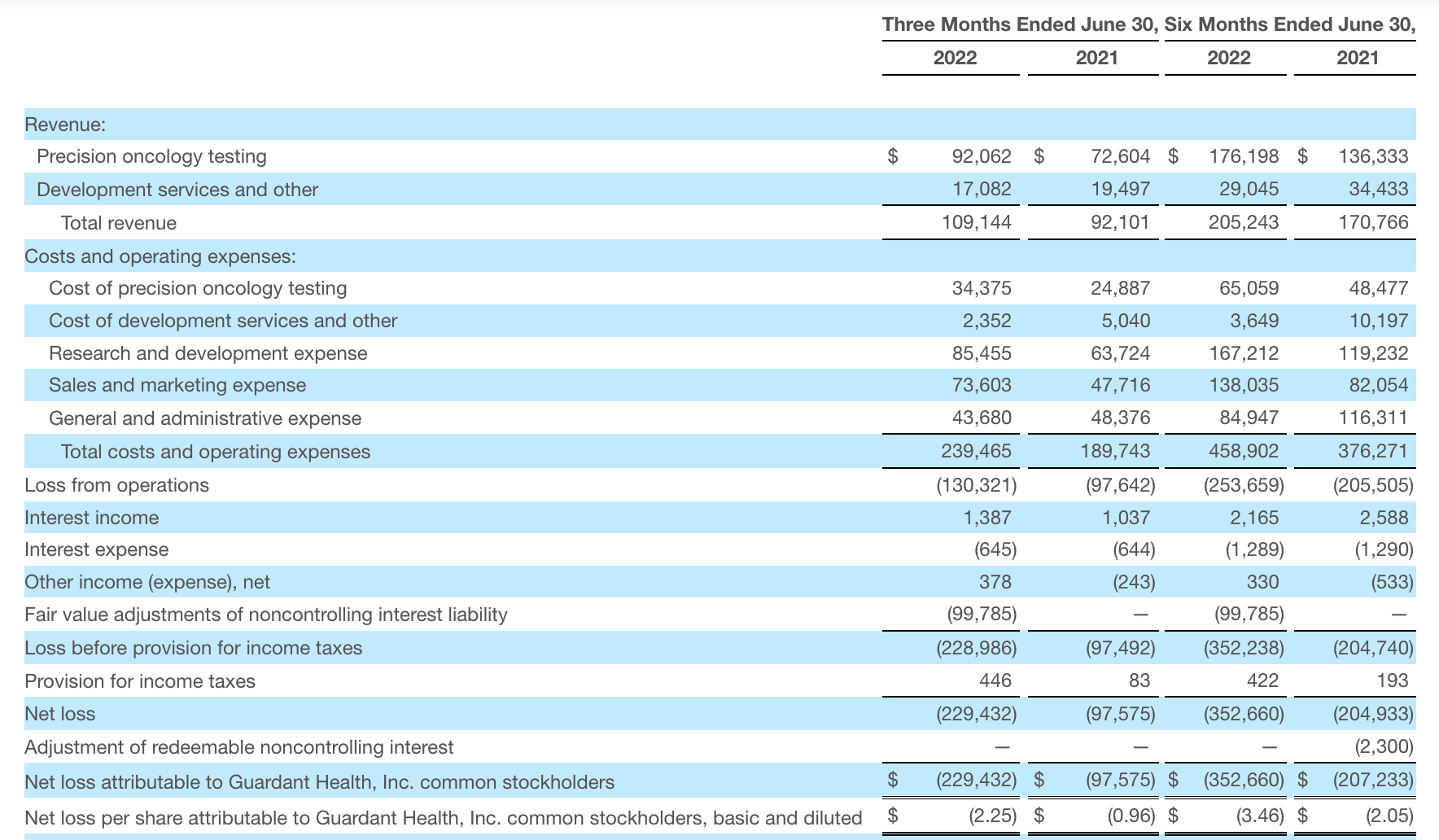
For the three months ended June 30, 2022, revenue was $109.1 million, an increase of 19% from $92.1 million for the three months ended June 30, 2021. Precision oncology revenue increased 27%, primarily driven by increased clinical testing volumes and biopharmaceutical sample volumes, which were up 40% and 65%, respectively, year over year.
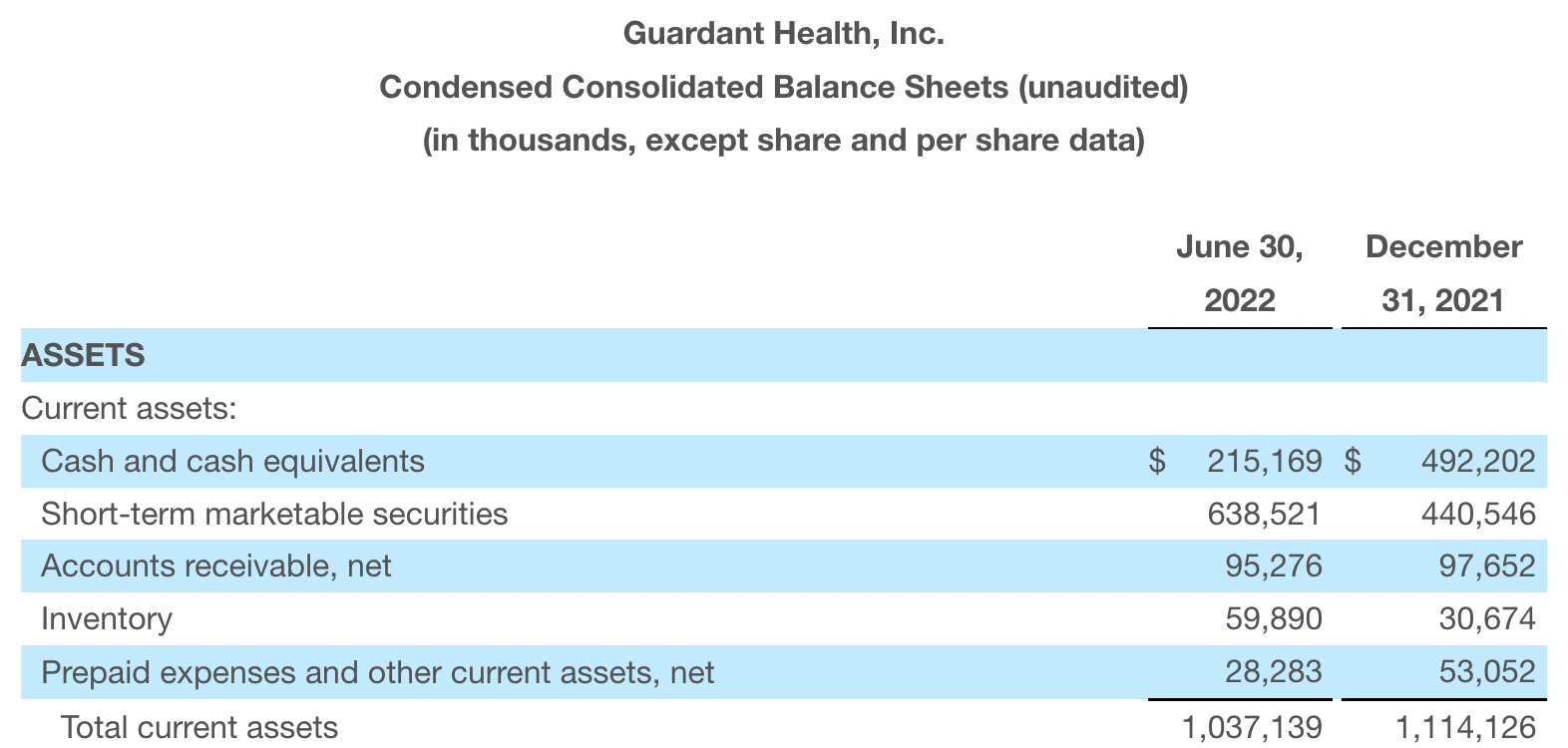
Cash, cash equivalents and marketable securities were $1.2 billion as of June 30, 2022. Of this, cash and cash equivalents were $215 million, compared with $492 million at the end of 2021. 2022 Q2 net loss of $229 million, compared to $97 million in the same period in 2021.
veracyte
Veracyte Announces Second Quarter 2022 Financial Results | Veracyte, Inc.
- Increased second quarter total revenue by 32% to $72.9 million, compared to the second quarter of 2021;
- Grew total test volume to 24,904, an increase of 19% compared to the second quarter of 2021;
- Announced that an updated clinical guideline from the American Urological Association and American Society for Radiation Oncology features a favorable statement for genomic testing, including Decipher Prostate, to help guide care for men with localized prostate cancer.
- Unveiled key clinical evidence across Veracyte’s portfolio:
- Decipher Prostate – Data was published in Annals of Oncology reinforcing the clinical utility of the Decipher Prostate genomic classifier for helping to guide the timing and intensity of therapy in men experiencing prostate cancer recurrence following radical prostatectomy. Additionally, data unveiled at the 2022 ASCO Annual Meeting demonstrated that population-based prostate cancer treatment patterns are independently associated with Decipher classifier score;
- Afirma Genomic Sequencing Classifier – Meta-analysis of independent, real-world studies were presented at ENDO 2022 demonstrating consistent and enhanced Afirma GSC performance, compared to the test’s original clinical validation study;
- Prosigna Breast Cancer Assay – New consensus survey data presented at the ESMO Breast annual meeting showed that leading breast cancer oncologists in Europe agree on the value of gene expression profiling tests, such as Prosigna, and on the importance of molecular subtype information to help inform treatment decisions for patients with early-stage breast cancer;
- Biopharma – New study findings presented orally at ASCO and in a paper published in Lancet Oncology showed the Immunoscore Immune Checkpoint (IC) biomarker’s ability to identify which patients will benefit from immune checkpoint inhibitors in metastatic non-small cell lung cancer and metastatic colorectal cancer, respectively; and
- Percepta Nasal Swab – Data presented at the ATS annual meeting showed that the noninvasive genomic test performed similarly well across the spectrum of tobacco-related risk.
- Ended the second quarter of 2022 with cash, cash equivalents and short-term investments of $164.0 million, compared to $166.4 million at the end of the first quarter of 2022.
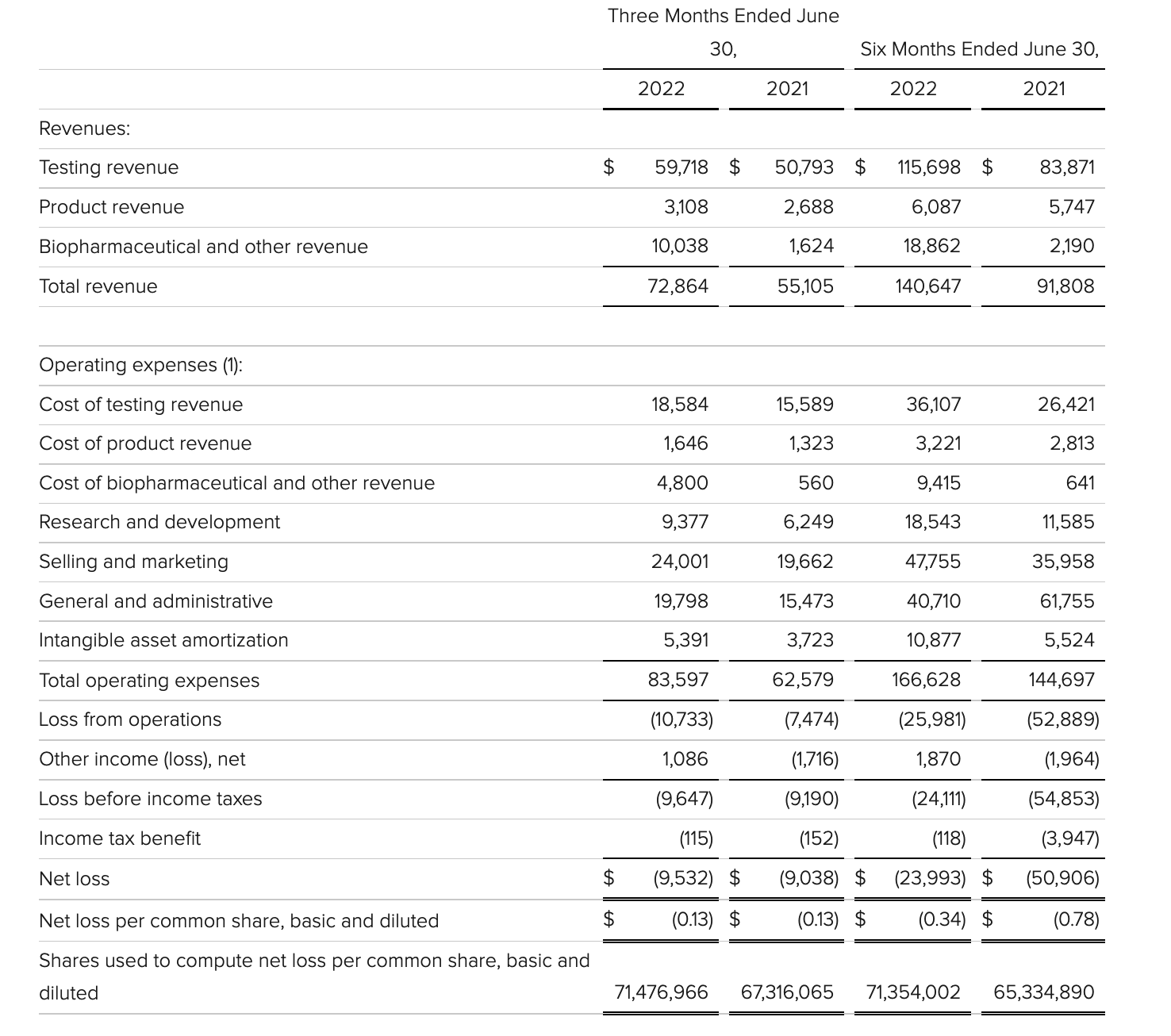
Total revenue for the second quarter of 2022 was $72.9 million, an increase of 32% compared to $55.1 million in the second quarter of 2021. Testing revenue of $59.7 million increased 18% compared to $50.8 million in the second quarter of 2021, primarily driven by strong performance in urology testing. Product revenue was $3.1 million, an increase of 16% compared to $2.7 million in the second quarter of 2021. Biopharmaceutical and other revenue was $10 million, an increase of $8.4 million compared to $1.6 million in the second quarter of 2021, primarily driven by contributions from the acquisition of HalioDx.
Veracyte posted a net loss of $9.5 million in the second quarter, compared with $9 million in the same quarter last year. Ended the quarter with $164.0 million in cash, cash equivalents and short-term investments. Total revenue for the full year 2022 is expected to reach $272 million to $280 million, a year-over-year increase of 24% to 28%.
Exact Sciences
Exact Sciences Corporation – Exact Sciences Announces Second Quarter 2022 Results
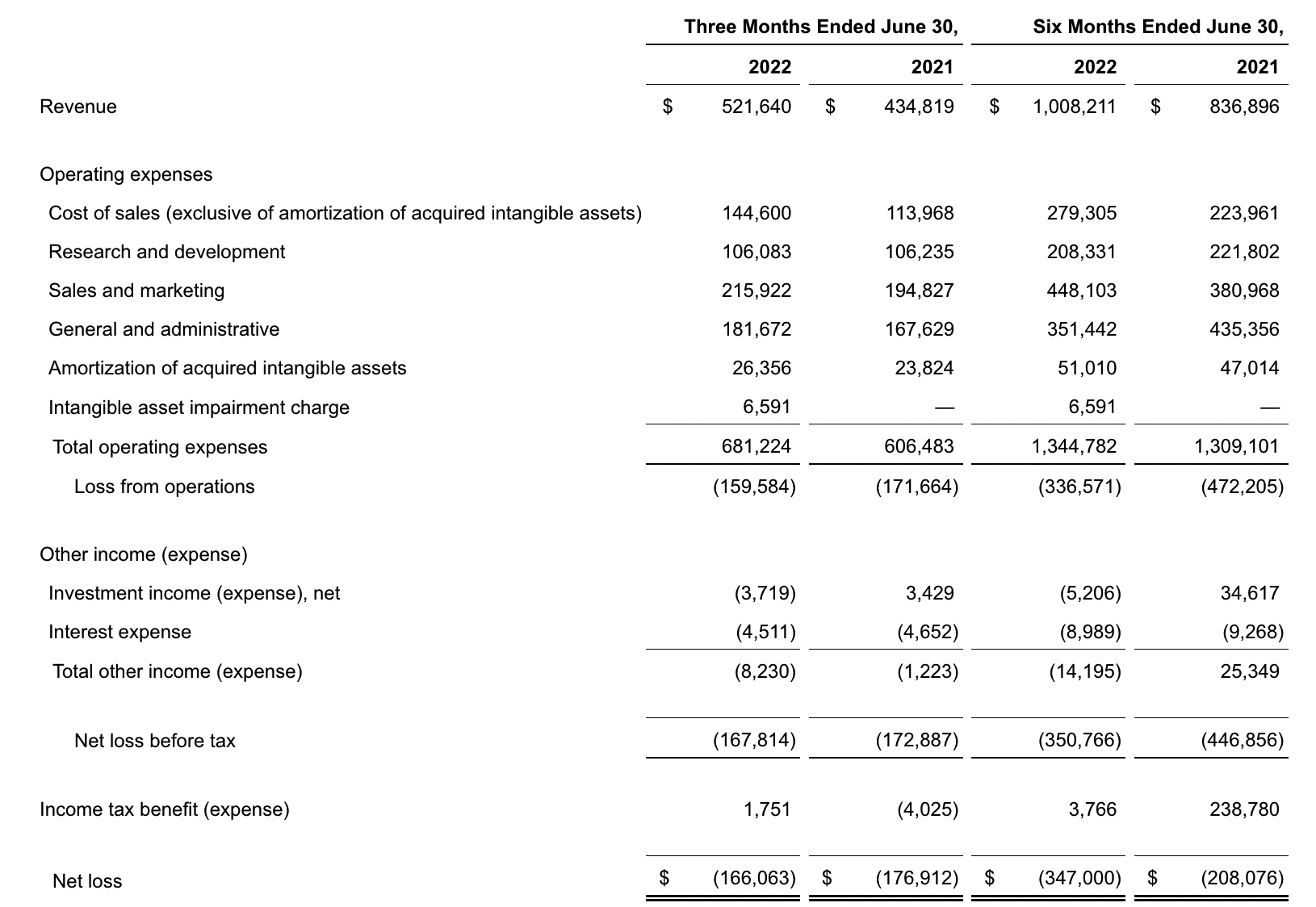
- Total second quarter revenue of $522 million, including Screening revenue of $354 million, Precision Oncology revenue of $154 million, and COVID-19 testing revenue of $14 million
- Total second quarter revenue, excluding COVID-19 testing, increased 26 percent compared to the second quarter of 2021, including 34 percent increase in Screening revenue and 12 percent increase in Precision Oncology revenue
- Screening revenue growth driven by improved sales team productivity, Cologuard marketing partnership with Katie Couric, Cologuard rescreens, and Cologuard use in 45-49 age group
The company’s total revenue for the second quarter was $521.6 million, up 20% from $434.8 million in the same period in 2021. Oncology-related revenue was $154 million, up approximately 12% year over year.
The company announced that it would drop the Oncotype DX test in the prostate to sell to MDx Health for up to $100 million.
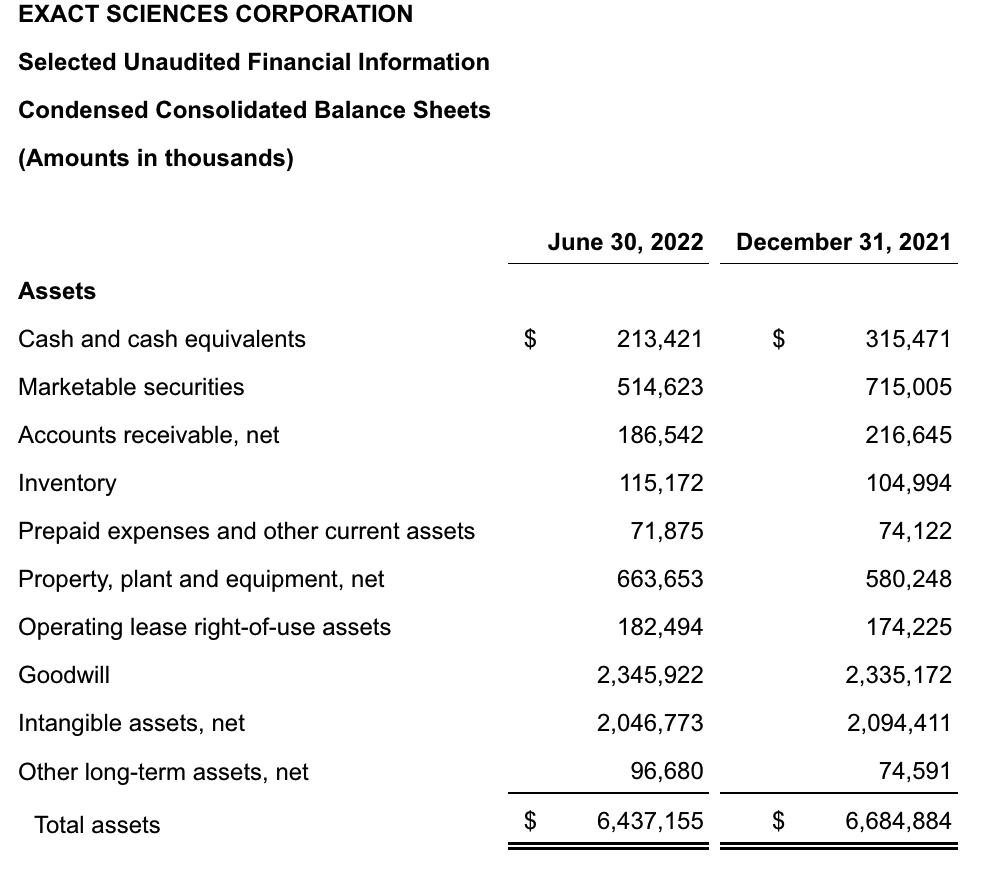
Exact posted a net loss of $166.1 million in the second quarter, compared with a net loss of $176.9 million a year earlier. The company ended the second quarter with $213.4 million in cash and cash equivalents and $514.6 million in marketable securities.
Invitae
Posted August 9, 2022
Burning Rock Medicine
Posted August 31, 2022
Generic
Unannounced time
The author of this article : Bear thinking about problems
Copyright notice : Unless otherwise stated, all articles on this blog are licensed under the Creative Commons Attribution-Non-Commercial-No Derivatives 4.0 International License Agreement (CC BY-NC-ND 4.0) .
This article is reproduced from: https://kaopubear.top/blog/2022-08-08-2022q2-ngs-precision-oncology-revenue/
This site is for inclusion only, and the copyright belongs to the original author.