Everyone already knows: the new crown star drug Paxlovid (Paxlovid) will be out of the 2022 medical insurance negotiations. As the world’s first FDA-approved oral drug for COVID-19, Pacloviride has never left the media spotlight since its birth. Pfizer also optimistically predicts that this drug is expected to bring Pfizer $22 billion in revenue in 2022 [1].
However, you may be wondering, how is this drug developed? Is it hard to make? Behind the high price, what is the cost? What other similar drugs are worth looking forward to?
The story of Parklowade may be quite old, let’s start with cats.
The “route problem” of treating cat diseases
Feline infectious peritonitis (feline infectious peritonitis virus), caused by the coronavirus FIPV (feline infectious peritonitis virus), was once an incurable disease for cats. At the beginning of this century, people began to study the use of a drug code-named GC376 to treat the disease.
GC376 is a 3CL protease inhibitor (3CL pro), its principle of action is: by inhibiting the coronavirus 3CL protease (3C-like protease), thereby inhibiting the formation of viral proteins, and ultimately making the virus synthesis and replication fail.
In 2018, Anivive, an animal protection company, bought patents from two researchers, Zhang Jingyu (장경옥/Kyeong-Ok Chang) and Kim Yunjeong (김윤정/Yunjeong Kim), from the School of Zoology at Kansas State University for global commercial development. [3]
However, the road to commercialization of GC376 is not smooth. The results of a trial in 2015 showed that after injection of GC376, only 7 of the 20 cats participating in the trial survived [4]; a study in 2020 showed that GC376 also needs to be combined with amodiaquine[ 5]. This means that the actual therapeutic effect of GC376 on FIP is mediocre.
As a result, GC376 quickly gave way to GS441524 (commonly known as GS441).
GS441 is a nucleic acid analog, its principle is to inhibit “RNA-dependent RNA polymerase” (RdRp), and its metabolites can competitively make viral RNA replication errors, and then terminate viral replication, referred to as RdRp inhibitors, for the treatment of feline diarrhoea. The effect is better. [6][7]
Although Gilead, the inventor of GS441, applied for a patent for the treatment of FIP for the drug in March 2018 (announced in June 2020, patent number US 20180296584 A1) [8], it is believed that the drug is more suitable for the treatment of Ebola Virus and human coronavirus infection, so the drug has also applied for the treatment of filovirus infection (patent number US 20180311263 A1) [9] and coronavirus infection (patent number US 20170071964 A1) [10]. However, Gilead has not produced and sold GS441 as a veterinary drug so far. As a result, this “magic drug” for cat belly transmission is still a sought-after commodity in the “black market” for cat owners. [11]
The two drugs for cat-loving humans to treat FIP also represent two technical routes against coronavirus: one is a 3CL protein inhibitor (GC376), and the other is a nucleic acid inhibitor, namely an RdRp inhibitor (GS441) . After the outbreak of the new crown epidemic, people also drew inspiration from these two technical routes.
Along the route of RdRp inhibitors, people found GS441’s prodrug GS5734, a molecule that also has an obvious effect on cat abdominal transmission. In 2020, it is called ” Remdesivir ” (Remdesivir), which is widely known; Merck & Co. Monogravir (Molnupiravir, or translated Monuprevir) is also an RdRp inhibitor. Practice has proved that neither remdesivir nor monupiravir have good anti-coronavirus effects.
Along the route of the 3CL inhibitor represented by GC376, Pfizer finally discovered the Paxlovid combination therapy.
The Birth of a Star New Drug
The development of Paclowade did not start from scratch, but was the result of Pfizer researchers rummaging through piles of old papers.
At the end of 2002, “SARS” (SARS) broke out, and global pharmaceutical companies also developed specific drugs for the SARS coronavirus. Among them, Pfizer developed a candidate drug in 2003, code-named PF-00835231. Just when they were preparing for clinical trials, SARS The outbreak was brought under control and development plans were put on hold.
Seventeen years later, the new crown epidemic broke out. In March 2020, while Pfizer and BioNTech were developing mRNA COVID-19 vaccines, they also started the development of oral drugs. The researchers found that the binding site between PF-00835231 and the “SARS” pathogen SARS-CoV also exists in the new coronavirus SARS-CoV-2, which means that PF-00835231 may be equally effective against the new coronavirus (tests have proved that it is true) , but the intravenous dosage form was too cumbersome, so Pfizer decided to transform it into an oral dosage form.
PF-00835231 is rich in hydrogen bonds and cannot be absorbed by the stomach after oral administration. Therefore, the difficulty is: to eliminate the hydrogen bond and ensure the activity of the drug without changing the antiviral properties of the drug. The Pfizer team boldly and cleverly used trifluoroacetamide to improve the bioavailability of the drug.
By July 22, 2020, a new candidate drug suitable for oral administration was born. On September 1, 2020, the results of the pharmacokinetic study of experimental rats were announced, and it was confirmed that the candidate drug code-named PF-07321332 was Pfizer’s oral drug. The final research results of the drug. [12]
PF-07321332 is named Nirmatrelvir.
On November 2, 2021, the Pfizer research team published a complete research paper on Pfizer’s oral drug PF-07321332 online in Science [13]; (Nirmatrelvir) combined with Ritonavir (Ritonavir), named Pacloviride (Paxlovid), applied to the FDA for emergency use authorization.
The principle of the combination is: Nematevir can inhibit the synthesis of new coronavirus proteins, but the drug will be rapidly degraded by an enzyme called CYP3A4 in the liver. At this time, it is necessary to use ritonavir to inhibit the activity of CYP3A4, so as to maintain the plasma concentration of nematevir at a certain level [14], so as to ensure the therapeutic effect twice a day.
On December 22, 2021, Pacloviride became the first oral drug for COVID-19 to receive emergency FDA approval.
On November 18 before approval, the US government ordered 10 million treatment courses from Pfizer at a price of US$5.29 billion [15]; on January 4, 2022, an additional 10 million treatment courses were ordered from Pfizer. [16]
Let’s do the math first: one box of Pacloviride is a course of treatment, which is a five-day oral dose, including five tablets (each tablet is for two doses a day), two 150mg tablets of Naimatevir and one Ritonavir 100mg. That is to say, each course of treatment needs to take 1.5g of nematevir and 1g of ritonavir. Therefore, the US government’s order for 20 million courses of treatment requires a total of 30 tons of Naimatevir. This is only the scale of the finished product, not the raw materials for all steps.
One mountain has passed one mountain block, and the next problem that Pfizer needs to solve is: how to mass-produce Pacloviride.
where is made in china
Is 30 tons of medicine too much?
In July 2020, the U.S. government placed an order with Pfizer for the first time to purchase mRNA COVID-19 vaccines, with a scale of up to 100 million doses [17]. By comparison, 20 million courses of oral medication doesn’t seem like a lot. But for Pfizer, this is obviously not easy.
After receiving the second order, Pfizer stated that even if it is accelerated, the first batch of 10 million orders will not be delivered until June 2022, and the second batch of 10 million treatment courses will be delivered in September [16]. During the 2022 JPMorgan Chase Pharmaceutical Annual Conference (JPM), Pfizer CEO Albert Bourla disclosed more detailed production capacity and delivery progress: 6 million to 7 million courses of treatment can be manufactured in the first quarter of 2022, and by the end of the second quarter of that year , the total output of Pacloviride will reach 30 million courses of treatment, and reach 120 million courses of treatment by the end of the year. [18]
What problems did the universe pharmaceutical company Pfizer encounter?
Pacloviride contains two ingredients: nymatrevir and ritonavir. The Guokehard technology team found that from the perspective of patent distribution and current market conditions, ritonavir is not difficult to manufacture.
Ritonavir was first developed by Abbott Life, a subsidiary of Abbott. In 1996, FDA approved ritonavir injection and capsules for the treatment of HIV infection [19]. In 2013, Abbott Life split into a new company Abbvie, which inherited the patent of ritonavir, produces the ritonavir tablet “Adgeway”.
In the early days of the COVID-19 pandemic, lopinavir-ritonavir (the anti-HIV combination drug “Kalitra”) was used in a COVID-19 treatment trial [20], but the effect was controversial. By October 2020, Oxford University, the British National Institute of Health and other institutions published a paper in The Lancet stating that randomized controlled, open-label parallel trials showed that Kaletra had no special effect on patients with new crowns. [twenty one]
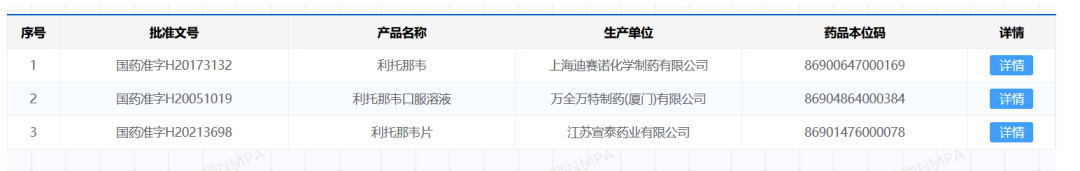
The patent of ritonavir has expired in 2017 [22], and many companies at home and abroad have produced related generic drugs. According to the official website of the State Food and Drug Administration of China, there are three domestic pharmaceutical companies that produce ritonavir raw materials (Shanghai Desano), oral liquid (Xiamen Wanquan Wante) and tablets (Jiangsu Xuantai).
Source丨National Food and Drug Administration official website
According to the announcement information, Ascletis Pharmaceutical alone produces ritonavir through Xuantai Pharmaceutical, with an annual production capacity of 530 million tablets[23]. The upstream of Xuantai Pharmaceutical comes from Senxuan Pharmaceutical , which has a designed production capacity of 230 tons per year. Supply chain personnel told Guoke Hard Technology that 70% of the ritonavir intermediate market is provided by Senxuan Pharmaceutical. Although the price of ritonavir has risen from 400 yuan/kg in 2021 to 11,000 yuan/kg by the end of 2022, the price is expensive, but the supply is not a problem.
Naimatevir is different. For global pharmaceutical companies, manufacturing Naimatevir is a brand new topic.
The Associated Press reported citing official Pfizer experts that the production of Pacloviride (mainly Naimatevir) is like building blocks, the key raw materials are located in different regions, and the manufacturers involve more than 20 locations in more than 10 countries. It takes 9 months and then shortens to 7 months. [twenty four]
The difficulties encountered by Naimatewei are not only cumbersome production steps, but the supply chain is also scratching the head. “Science” columnist Derek Lowe pointed out that there may be many problems in this production chain:
Many production steps involve the Boc group, that is, di-tert-butyl dicarbonate (DIBOC), but the capacity of DIBOC is not so much, because its upper raw material is sodium tert-butoxide, which requires tert-butanol and metal Sodium synthesis. [25]
This is the upstream supply chain bottleneck encountered by most countries, including the United States: there is not enough metallic sodium .
Producing sodium metal requires a large-scale electrowinning industry. In 2020, the global sodium metal production capacity will be about 160,500 tons. Among them, the capacity of Chinese installations is 132,500 tons. The sodium metal production capacity of China Salt Chemicals alone reached 65,000 tons, accounting for 40.5% of the global production capacity. [26]
“Basically, if you go back into the production of any drug, right down to the earliest stages, you almost always find a bunch of offshore suppliers. They are mostly Chinese, though there are some Indians and other nationalities as well.” In his op-ed, Crowe pointed out that “there is no such capability in the United States anymore, because it is much cheaper to let someone else do it.”[25]
Therefore, if Naimatevir must be manufactured according to Pfizer’s published process roadmap, then China must become the core origin of Naimatevir’s upstream raw materials . However, the upstream raw materials may be in China, but the drug precursors and intermediates are not necessarily. People in the supply chain told Nutshell Technology that the main raw material base of Naimatewei is in Europe.
So, what is the supply of raw materials and finished products in China?
“Miraculous medicine” is difficult to make
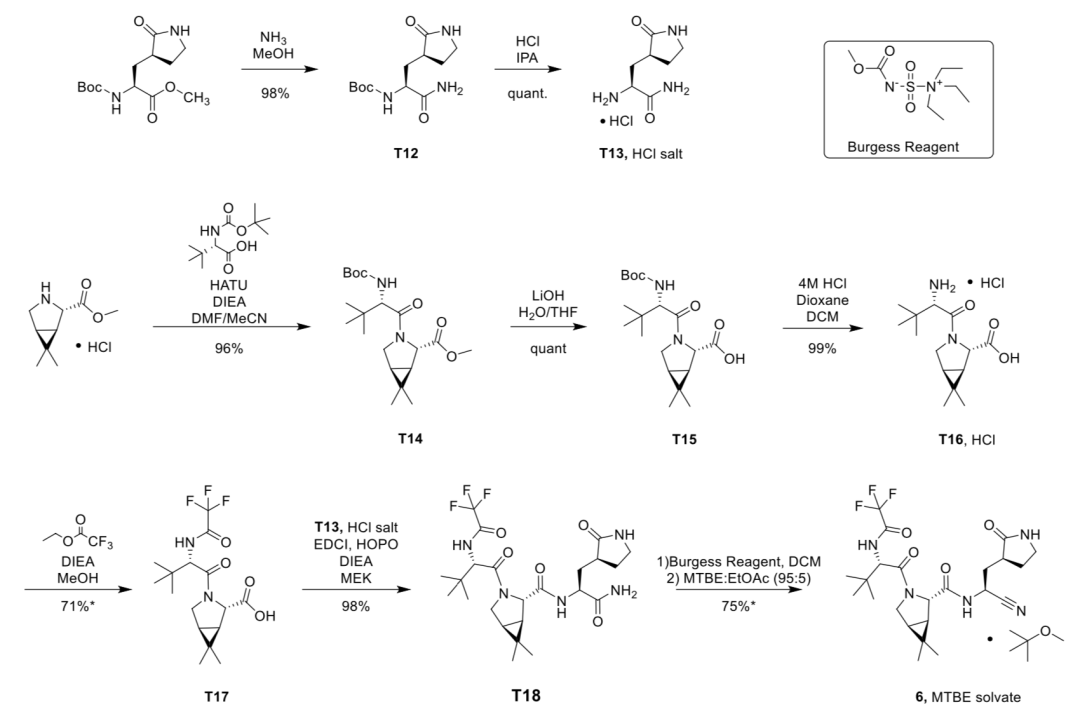
The paper published by Pfizer in the “Science” magazine shows that the company synthesized 150g of Naimatewei in the laboratory. The following figure is the method given in the paper, which requires at least 8 steps——
Source丨Science[5]
According to the above steps, the key raw materials include the following:
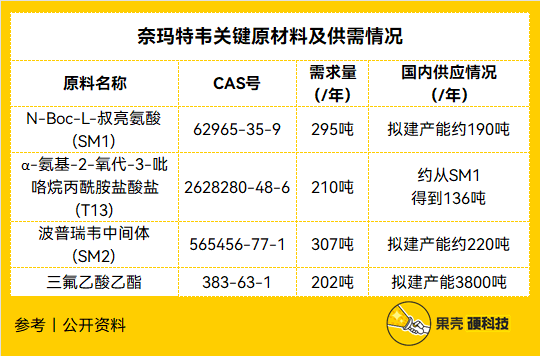
Tabulation 丨 Nutshell Hard Technology Team, data source 丨 Guotai Junan Securities [27] According to Pfizer’s demand for 120 million treatment courses in 2022, the key raw materials and supply and demand of Naimatevir (PF-07321332) are estimated
It can be found that according to Pfizer’s planned production capacity of 120 million treatment courses in 2022, the supply of raw materials in China alone can meet more than 60% of the demand. A small amount of supply gap is concentrated in the key raw materials SM1 and SM2, which will be discussed separately below.
Let’s look at SM1 first. The research report of Minsheng Securities pointed out that the total yield of manufacturing gram-level SM1 is 86%, which is higher than 66% of the production of kilogram-level, but the industrialization is more difficult, so the production practice still needs to use the kilogram-level manufacturing process. The latter requires low-temperature and bromoacetonitrile raw materials produced at the industrial level at -120°C, so the production difficulties lie in ultra-low temperature production conditions and qualification barriers for cyanide raw materials. [28]
However, a person in the chemical industry who requested anonymity told the Guoke Hard Technology Team (ID: guokr233) that in chemical parks, large pharmaceutical companies usually have a good relationship with the local government. “For projects such as new crown specific drugs, the local government will give green light all the way, and there will be no major problems in environmental assessment and safety assessment.” Therefore, if necessary, economic interests should be able to drive domestic suppliers to fully make up for the existing gap, and then meet T13 Yield.
Look at SM2 again. The production difficulty of SM2 is less than that of SM1. Although the current direct supply is insufficient, according to the total demand of Nematervir this year, the demand for SM2 intermediate carronic anhydride is about 454 tons per year. The current supply of this raw material is 270 tons/year, and the proposed capacity exceeds 1,000 tons[27]. That is to say, there is basically no supply problem for SM2, it only depends on when the production line under construction will be mass-produced.
Of course, the above reaction yield and supply chain demand are only calculations made by relevant research institutions based on Pfizer’s official documents. These are ideal conditions, and the actual production process may be quite different.
A chemical engineering researcher from a university told the Guoke Hard technology team that the current domestic public opinion is based on the paper published by Pfizer in November 2021 to speculate and piece together the supply chain of Naimatevir, and the actual production process may be different from the process given by Pfizer. There are differences, and it does not rule out the possibility that Pfizer may have developed a better process.
“The yield given by scientific research papers cannot be fully trusted.” The above-mentioned anonymous industry insider also pointed out that the raw materials actually used by the patent applicant may be hidden in vague descriptive sentences such as “including but not limited to ××”. According to the patent specifications of the chemical industry to imitate products, the effect is not good enough. “Under normal circumstances, the actual production process will not be given in patents and papers for the products you want to produce. The verification data such as NMR and mass spectrometry in the paper are true, but the key techniques in the synthesis process (such as segmental heating) , addition sequence, etc.) may be concealed, especially for literature that does not focus on the synthesis process .” Literature usually gives a condition that can achieve a synthetic route, and R&D personnel can generally achieve higher yields through optimization. Rate.
“The process route in the process of making medicines may be very different from the process route in the final production in the factory.” The aforementioned chemical engineering researcher in a university said that the process of chemical drugs will be “rollingly optimized” from research and development to production. He told Guoke The hard science and technology team, “The ultimate goal is to achieve a large number of reproducible synthesis at the cheapest possible price, with the highest possible yield, and with the shortest possible steps.”
At present, many domestic companies are already trying to optimize the production process of Nematevir intermediates. According to the official website of the State Intellectual Property Office, many Chinese companies’ patents for the optimization process of Nematevir are in the application period.
In short, there is no need to doubt the strength of China’s production of Naimatevir, but non-financial practitioners in the chemical industry can only discuss the supply chain through public information, and they should be more cautious in guiding investment.
However, if sufficient raw materials are supplied, can production go smoothly and be supplied in a timely manner?
Skinny 3CL
The story of 3CL protease inhibitors started with cats and became famous after Naimatevir. The road of 3CL inhibitors is crowded with pharmaceutical companies gearing up, making the research and development of 3CL new crown drugs particularly crowded.
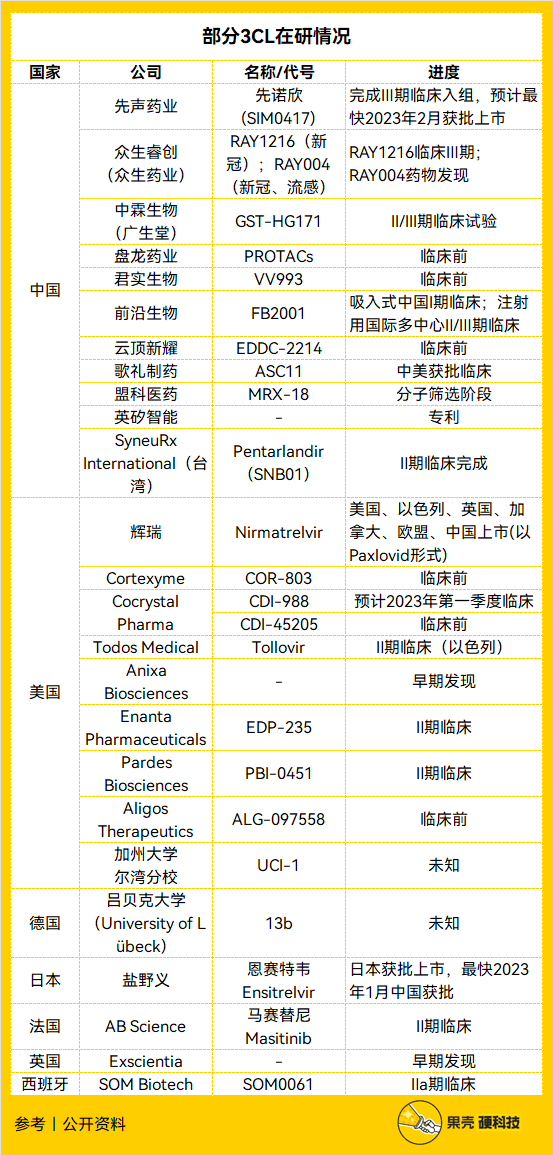
Here is the incomplete statistics of the 3CL new drug under research by the Guokehard technology team——
Tabulation丨Guokehard technology team, source丨public information
In fact, Pfizer has simultaneously developed another 3CL protein inhibitor, Lufotrelvir (the phosphate prodrug of nematervir, code-named PF-07304814), but Pfizer’s annual report shows that the study has been terminated in February 2022.
The research and development progress of the 3CL new crown drugs in the above table is very fast. For example, Shionogi’s 3CL new crown drug Ensitrelvir (Ensitrelvir, code name S-217622, trade name Xocova) was approved by the Ministry of Health, Labor and Welfare of Japan on November 22, 2022. License to use. [29] It has become the second 3CL new crown drug in the world, and the application has been submitted in China, waiting for the approval of the Food and Drug Administration.
Domestic Simcere Pharmaceuticals and Zhongsheng Pharmaceuticals have started Phase III clinical trials, both of which have the same advantages as Shionogi: they can be directly administered as a single drug without the need for ritonavir. According to news, Simcere Pharmaceuticals’ 3CL new crown drug will be approved in February 2023. In 2023, we are expected to see at least four 3CL new crown drugs in the Chinese market.
Market competition is imminent. Although Pfizer expects to sell $22 billion of Pacloviride in 2022, the performance does not necessarily count with a calculator.
Pfizer has been looking for OEMs in China to place orders, and has also signed agreements with MPP to authorize 36 pharmaceutical companies in low- and middle-income countries to carry out generics [30].
The truth is — luck doesn’t always accompany Pfizer.
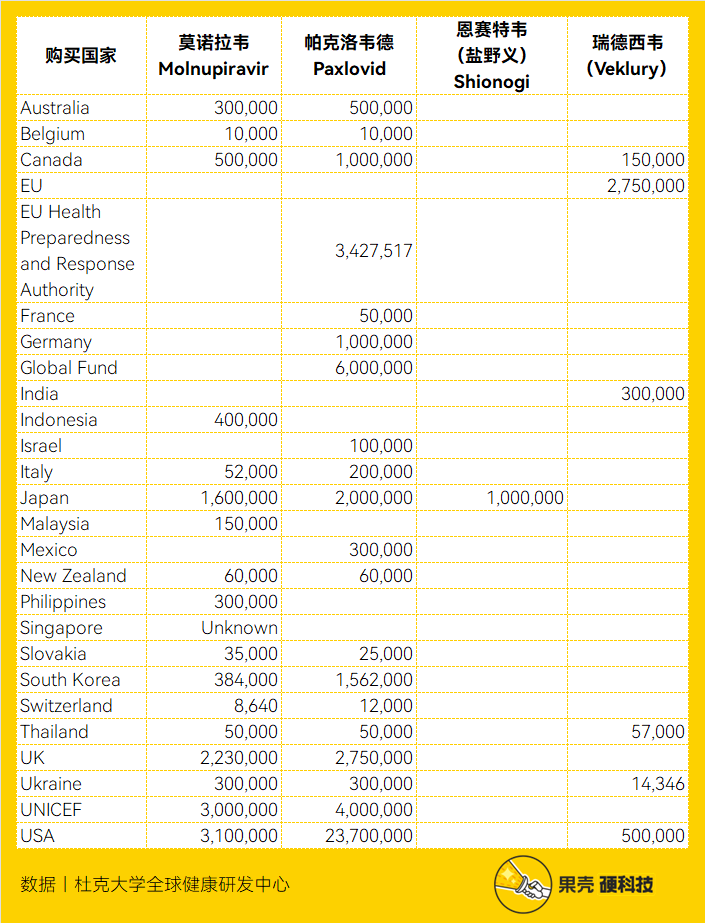
Because it can reduce the hospitalization rate of high-risk patients by 89% and the mortality rate by 88% [31], in the first quarter of 2022, all parts of the world are looking forward to Pacloviride. According to data from Duke University’s Global Health Innovation Center, many countries around the world have ordered Paclovir from Pfizer. As of January 6, 2023, the total number of Pacloviride orders worldwide exceeds 48.186 million courses of treatment. [32]
As of January 6, 2023, the number of new crown oral drugs purchased by countries, source丨Duke University Global Health Innovation Center[32]
According to data from the official US platform HealthData, as of April 22, 2022, 19,207 pharmacies across the United States can provide a total of 632,825 courses of Pacloviride. By January 2023, 97,481 pharmacies across the United States can provide a total of 2,635,353 courses of treatment. [33]; Although South Korea has ordered 762,000 courses of treatment, more than 9.7 million people will be infected with the new crown from January to March 2022, and only 113,783 people will take the drug during the same period [34]; clinical research institutions in Africa are asking Pfizer for When experimenting with drugs, they were rejected directly. [35]
It seems that Pfizer’s new crown oral medicine is in short supply. However, how much you order is one thing, and how much you get is another matter. Multiple factors have affected the sales growth rate of Pfizer’s new drug.
First, it’s too expensive.
Regeneron cocktail antibody therapy REGEN-COV (casirivimab+imdevimab) is priced at $2,100/dose, and Merck’s oral drug Molnupiravir is priced at $700/course. Compared with these expensive new crown drug predecessors, Pfizer Parker Lowe’s price is US$529 per course of treatment (according to the US government’s first batch purchase price), which is relatively “cheap” [37], but it is still unbearable for ordinary people, and the government has to weigh it before purchasing.
As evidenced by government order data, according to the statistics of Duke University’s Global Health Innovation Center, by January 6, 2023, the U.S. government, the world’s largest orderer, has a total of 23.7 million courses of treatment, compared with 20 million courses of treatment in April 2022 , an increase of 3.7 million treatment courses in 9 months; followed by 2.75 million treatment courses in the United Kingdom, compared with April 2022, the number of orders ordered by the British government has not changed.
As long as Pfizer insists on a high price, Parklowerd’s sales numbers won’t look good. It is worth mentioning that Charles Gore, executive director of the Medicines Patent Pool (MPP), estimates that the cost of Pacloviride is only US$20-25 per course of treatment, and may drop to US$10 per course of treatment in the future . [38]
Second, the medication requirements are somewhat special.
In the instruction manual, Pfizer recommends that patients take Pacloviride within five days of symptom onset after diagnosis [39], but NPR quoted health experts as pointing out that before prescribing, doctors should consider whether patients’ commonly used drugs interact with Pacloviride. effects (for example, ritonavir can cause liver damage [40]), which prevents some patients from taking their medications as required.
Even if patients meet the medication standards, they may not be able to get a prescription because they cannot make an appointment with a doctor, or the nearby pharmacy is out of stock when they get a prescription, resulting in the embarrassment of “not being used when not in use, and idle when not in use”. [41]
In an article in January 2023, “Nature” magazine quoted a report from Airfinity, a medical data agency, saying that in the UK, doctors only prescribed Paclovir for about 0.5% of patients with new crowns, while in the United States, this proportion was about 13%. %. [42]
Third, the production capacity is really not going up.
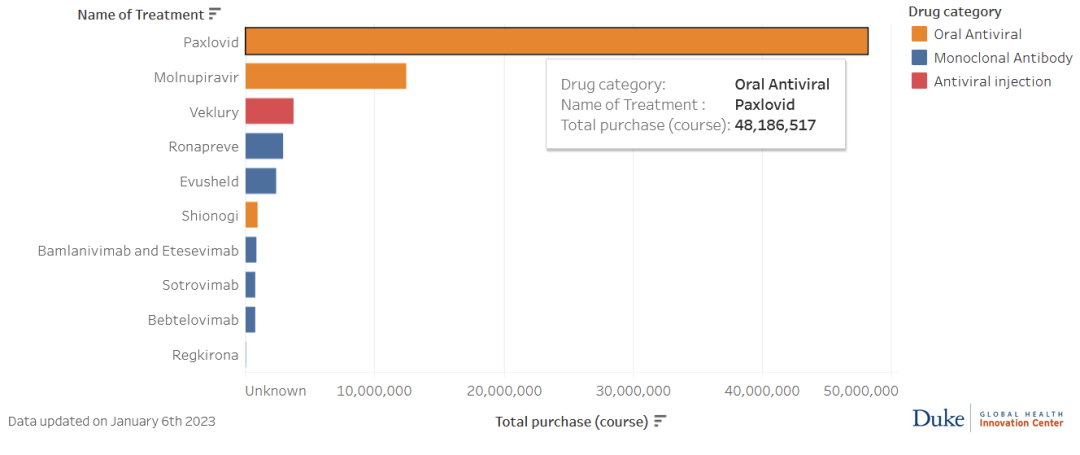
Pfizer started preparations for production capacity in June 2021, and produced the first batch of Pacloviride in September 2021[24]. According to data from Duke University’s Global Health Innovation Center, by early April 2022, the total order volume of Pacloviride was 28,822,000 courses of treatment, and by early January 2023, the order volume reached 48,186,517 courses of treatment. [32]
As of January 6, 2023, sales of various new crown oral drugs and monoclonal antibody drugs, source丨Duke University Global Health Innovation Center[32]
Although Pfizer has sent orders to several Chinese CDMO companies, and MPP has also authorized 36 companies to carry out generics[31], but in the first half of 2022, Pacloviride will be mainly sold to rich countries (and not enough to sell), as for generic drugs , according to estimates by Doctors Without Borders, may not be available until 2023. [43]
But that’s presumably what Pfizer was aiming for.
From ramping up production capacity, to price, to global distribution, Parklowade will repeat the story of vaccines: Technology can only watch the world fall into a crisis due to huge differences in economic strength, medical resources, company interests and cognitive levels. Another round of inequality, powerless to change.
The end is not yet reached
For humans trapped in the quagmire of the epidemic, Parklowade brought hope. However, the emergence of Pfizer’s new crown oral drug is not the end. We still need to cut off the route of infection (pay attention to hygiene, social isolation), protect the susceptible population (vaccination), and manage the source of infection (timely admission). Prepare enough ammunition to fight against the epidemic comprehensively.
Only by uniting and returning to common sense can mankind usher in the real dawn.
References
[1] Pfizer 2021 fourth quarter and full year financial report https://ift.tt/atpq57f
[2] FDA: FDA Authorizes First Oral Antiviral for Treatment of COVID-19 https://ift.tt/BXGRjgm
[3] Veterinary researchers and Anivive license antiviral drug for fatal cat disease 2018-9-20 https://ift.tt/UXt6Ipo
[4] Kim, Yunjeong; Shivanna, Vinay; Narayanan, Sanjeev; Prior, Allan M.; Weerasekara, Sahani; Hua, Duy H.; Kankanamalage, Anushka C. Galasiti; Groutas, William C.; Perlman, S. (2015). Broad-Spectrum Inhibitors against 3C-Like Proteases of Feline Coronaviruses and Feline Caliciviruses. Journal of Virology, 89(9), 4942–4950. doi:10.1128/jvi.03688-14
[5] A rational approach to identifying effective combined anticoronaviral therapies against feline coronavirus. SE Cook, H. Vogel, D. Castillo, M. Olsen, N. Pedersen, BG Murphy. bioRxiv 2020.07.09.195016; doi: https://ift .tt/QoUFZ6w
[6] BG Murphy, M. Perron, E. Murakami, K. Bauer, Y. Park, C. Eckstrand, M. Liepnieks, NC Pedersen, The nucleoside analog GS-441524 strongly inhibits feline infectious peritonitis (FIP) virus in tissue culture and experimental cat infection studies, Veterinary Microbiology, Volume 219, 2018, Pages 226-233, https://ift.tt/VksgB5b.
[7] Pedersen NC, Perron M, Bannasch M, et al. Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis. Journal of Feline Medicine and Surgery. 2019;21(4): 271-281. doi:10.1177/1098612X19825701
[8] USPTO document: https://ift.tt/9xSQvYG
[9] USPTO document: https://ift.tt/VyrA2kZ
[10] USPTO document: https://ift.tt/wAfny73
[11] Sarah Zhang: A Much-Hyped COVID-19 Drug Is Almost Identical to a Black-Market Cat Cure, 2020-5-8 https://ift.tt/WZN0AdU
[12] Bethany Halford: How Pfizer scientists transformed an old drug lead into a COVID-19 antiviral. 2022-1-14 https://ift.tt/hytbJo5
[13] An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19 https://ift.tt/KJuIoYi
[14] NIH: Ritonavir-Boosted Nirmatrelvir (Paxlovid) 2022-2-24
[15] Pfizer to Provide US Government with 10 Million Treatment Courses of Investigational Oral Antiviral Candidate to Help Combat COVID-19 https://ift.tt/eEngGVy
[16] Pfizer to Provide US Government with an Additional 10 Million Treatment Courses of its Oral Therapy to Help Combat COVID-19 https://ift.tt/WrcPqeH
[17] Pfizer and BioNTech Announce an Agreement with US Government for up to 600 Million Doses of mRNA-based Vaccine Candidate Against SARS-CoV-2 https://ift.tt/KYpNSm6
[18] JPM 2022: Pfizer CEO eyes 50% boost to Paxlovid production—and capacity expansions, toohttps://https://ift.tt/K6X8eTi
[19] FDA approval to Abbott: https://ift.tt/FGm0EQV
[20] Arabi, YM, Asiri, AY, Assiri, AM et al. Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon-β1b ( MIRA CLE trial): statistical analysis plan for a recursive two-stage group Sequential randomized controlled trials. Trials 21, 8 (2020). https://ift.tt/SgL3qHT
[21] RECOVERY Collaborative Group. Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. The Lancet, doi: 10.1016/S0140-6736(20)32013- 4
[22] Intellectual Property Development Research Center of the State Intellectual Property Office: Analysis of the Patent Layout of Antiviral Drugs of AbbVie Company, the Original Drug Company of Lopinavir/Ritonavir 2020.2.26 https://ift.tt/0Le4pD5
[23] Ascletis Announcement: Ascletis announced that the annual production capacity of ritonavir oral tablets will be further expanded to 530 million tablets https://ift.tt/PtjB6Mw
[24] Tom Murphy: EXPLAINER: Why Pfizer needs time to make COVID-19 treatment 2022-3-2 https://ift.tt/OZqSFKg
[25] Derek Lowe: Making Paxlovid https://ift.tt/jmIPTLH
[26] China Salt Chemical Industry 2020 Annual Report https://ift.tt/upZeRCd
[27] Guotai Junan Securities 2022 Spring Strategy Seminar: Focusing on Comparative Advantages under Rigid Demand
[28] Minsheng Securities: The authorization of MPP’s new crown generic drugs has started, and the upstream is expected to accelerate the increase in volume
[29] Kyodo News: Shionogi’s new crown oral drug may cause teratogenicity and is not recommended for pregnant women to take it 2022-4-13 https://ift.tt/zBSDoYa
[30] Update: Ukrainian company Darnitsa signs sublicence agreement with MPP bringing to 36 the number of generic manufacturers to produce generic versions of Pfizer’s oral COVID-19 treatment https://ift.tt/jL6WpfN
[31] Pfizer Announces Additional Phase 2/3 Study Results Confirming Robust Efficacy of Novel COVID-19 Oral Antiviral Treatment Candidate in Reducing Risk of Hospitalization or Death https://ift.tt/Jz9BiHX
[32] Launch and Scalefaster: COVID-19 THERAPEUTICS https://ift.tt/qlWE69v
[33] HealthData: COVID-19 Public Therapeutic Locator https://ift.tt/yboIw5F
[34] Kim Arin: Amid Paxlovid scarcity, Korea looks for alternatives 2022-3-25 https://ift.tt/YPpbtVl
[35] African clinical trial denied access to key COVID drug Paxlovid. Nature 604, 412-413 (2022) https://ift.tt/zCpfPn4
[37] Deena Beasley: Price of COVID treatments from Pfizer, Merck, GSK align with patient benefits -report https://ift.tt/WzxrVAR
[38] Jenny Lei Ravelo: 35 companies in 12 nations to manufacture Pfizer’s oral COVID-19 drug https://ift.tt/CQiMsdc
[39] Pfizer Official: Fact Sheet for Healthcare Providers: PAXLOVID  Emergency Use Authorization https://ift.tt/yOsY60N
Emergency Use Authorization https://ift.tt/yOsY60N
[40] FDA: EMERGENCY USE AUTHORIZATION (EUA) OF PAXLOVID FOR CORONAVIRUS DISEASE 2019 (COVID-19) https://ift.tt/irgtd0V
[41] Pein Huang: Lifesaving COVID drugs are sitting unused on pharmacy shelves, HHS data shows https://ift.tt/VTu9IRh
[42] COVID drug Paxlovid was hailed as a game-changer. What happened? doi: https://ift.tt/bx6MC8L
[43] MSF responds to Medicines Patent Pool deal with 35 manufacturers to produce COVID-19 treatment nirmatrelvir/ritonavir https://ift.tt/CK3DpY5
This article comes from the WeChat public account “Guokrhard Technology” (ID: guokr233) , author: Li Tuo, Yang Jingyi, editor: Li Tuo, 36 Krypton is authorized to publish.
media reports
36Kr Sina The Paper News Tencent Technology Sina
Event Tracking
- 2023-01-10 Decoding Paxlovid: There is no magic drug, only the magic drug
- 2023-01-08National Medical Insurance Administration: Paxlovid failed to be included in the medical insurance catalog through negotiation due to Pfizer’s high offer
- 2022-12-13 Online shopping for Paxlovid, a special drug for the new crown, opens, and you can buy it based on nucleic acid or antigen results
- 2022-02-12 State Food and Drug Administration emergency conditional approval of Pfizer’s new coronavirus treatment drug
- 2021-11-05 Pfizer says its antiviral drug Paxlovid can significantly reduce COVID-19 hospitalizations and deaths
This article is transferred from: https://readhub.cn/topic/8msih5AjXpU
This site is only for collection, and the copyright belongs to the original author.