36氪 was informed that the biotechnology company [Jiajin Bio] recently completed an angel round of financing of tens of millions of yuan. The company focuses on the development of extrahepatic targeted small nucleic acid conjugates (X-oligo conjugate, XOC). This round of financing The investment was jointly led by Matrix Partners and Fengrui Capital, and the funds obtained were used for pipeline research and development, patent layout, company operations including laboratory platform construction, etc.
At present, the Jiajin biological team covers 4 core R&D functional areas, including nucleic acid chemistry, biological conjugation, drug analysis and cell biology; in addition to R&D, the team also includes talents in project management and BD. Company founder/CEO Wu Hao graduated from Tsinghua University with a bachelor’s degree and a Ph.D. from the School of Pharmacy, University of Tennessee. He has worked for NGM, Novo Nordisk, WuXi Biologics, Deerfield, Tonghe Yucheng, and has experience in drug R&D and industrialization. Rich.
It is understood that the first product of Jiajin Bio has nearly completed the PCC (preclinical candidate compound) screening, and it is expected to start the IND-related work in 2023. Recently, in an interview with 36氪, Dr. Wu Hao described the original intention of Jiajin Bio-focused small nucleic acid conjugated drugs, the research and development progress, and the industry trends in this field.
Characteristics of Small Nucleic Acid Antibody-Drug Conjugates
At present, ADC (antibody-conjugated drug) is developing rapidly in China. As of December 2021, there are 74 ADC drugs in different research and development stages in China, of which nearly 1/3 of the drugs target HER2 targets, and the pipeline of companies under development There is a high degree of overlap and competition is fierce.
Around 2017, when Wu Hao served as the preparation director of WuXi Biologics, he successively came into contact with a number of domestic ADC projects, and the current team also has experience in developing ADC drugs. But in the face of homogeneous competition, “At the beginning of our business, we were thinking about how to make something different. A key idea was to find new payloads and stop being addicted to toxins,” Wu Hao said. express.
Based on this idea, combined with the great potential shown by small nucleic acid drugs in recent years, Jiajin biological team considered if the tissue-specific advantages of antibodies, peptides, etc. could be combined with the target-specific advantages of small nucleic acids, small nucleic acids could be developed. Conjugated drugs to solve the current problem that small nucleic acid drugs can only target the liver through LNP (lipid nanoparticles) and GalNAc (N-acetylgalactosamine) delivery systems, while avoiding the risk of ADC drugs due to accidental release of toxins, This new therapy will bring great value.
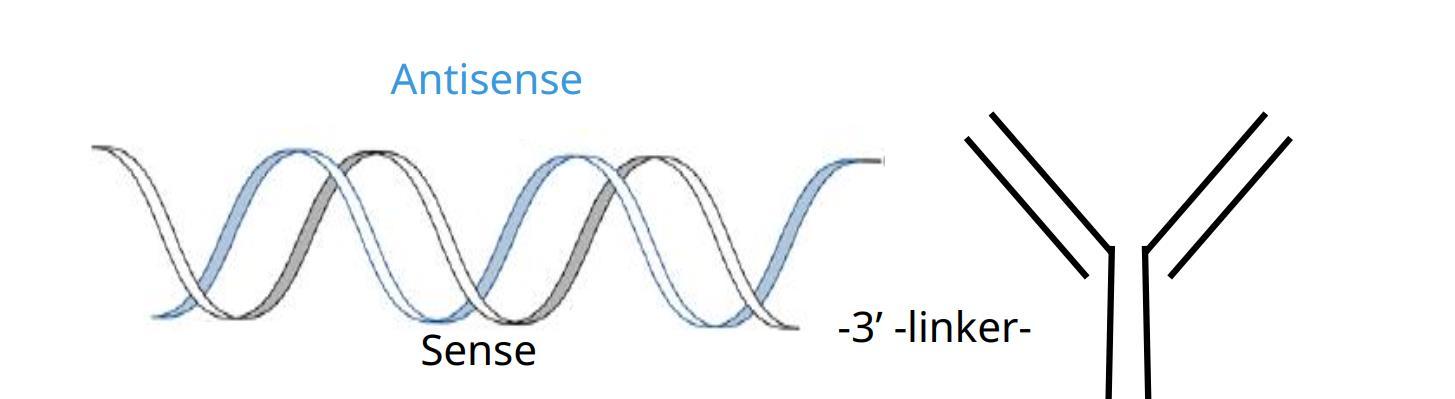
The structure of small nucleic acid-antibody-drug conjugates is similar to that of ADC, and is mainly composed of three parts: a carrier that plays a role in tissue targeting, a linker, and a small nucleic acid that acts as a payload. The difference is that ADC usually uses small toxin molecules as payload, while the molecular weight of small nucleic acid used in XOC is usually above 10kDa, which is much higher than that of ADC; in addition, small nucleic acid is negatively charged in the case of neutral pH, so it is not suitable for coupling with antibodies. The process can present unique challenges.
The structure of the small nucleic acid conjugated drug, photo courtesy of the company
To develop small nucleic acid conjugated drugs, we must first identify a tissue-specific target to achieve a targeted delivery mode similar to ADC. At present, in non-liver tissues, some targets of neuromuscular organs have been disclosed, and the progress is relatively fast Avidity Biosciences and Dyne Therapeutics are developing muscle-targeted drugs for this target, such as TfR1; Denali Therapeutics is trying to select peptides with affinity as carriers to achieve delivery across the blood-brain barrier and treat the CNS center. Nervous system disease.
Jiajin biological research and development progress
In terms of indication selection, Jiajin Bio currently mainly develops drugs for genetic diseases of the neuromuscular system. First, because the TfR1 target has been verified, and in the targeted delivery of muscle organs, high-affinity antibodies are often required to achieve , and ideal antibody molecules can be obtained through affinity screening; the second is that in the field of muscle diseases, there are still a large number of genetic rare diseases that have not yet had effective treatments, and genetic diseases targeting single gene mutations are used as payloads. Small nucleic acid drugs have the function of “correcting” faulty genes, which can effectively solve this problem.
After 10 months of research and development, Jiajin Bio has made a series of progress in the design and screening of small nucleic acid molecules, antibody discovery, and AOC coupling technology:
First, a satisfactory payload has been obtained. Through in vitro experiments, it is found that the biological activity of Jiajin Bio’s candidate siRNA is about 5 times higher than that of the competitor’s leading molecule.
Second, in the research and development of patented antibodies, through cooperation with external CRO companies, the animal immunization and screening work has been completed, and some hit antibodies have been obtained, which are expected to enter the humanization stage in December; The exchange experiment identified the antigenic epitope for the first time, which will be of great value for future antibody patent protection.”
Third, at the coupling process level, different from random coupling, Jiajin Bio-selected site-specific coupling combined with click chemistry technology can realize the coupling site at the fixed position of the antibody; and there are fewer purification steps, only one purification step is required ; The control of the molecule is relatively uniform; the affinity for the antigen will also be higher than that of the random coupling technology. It is understood that Jiajin Bio has completed the production of the first batch of candidate molecules, and the candidate molecules are currently undergoing animal testing.
Jiajin Bio is positioned as a biotech company, and its core pipeline mainly targets two indications, DM1 and DMD. In terms of user population and market size: DM1 is a genetic disease related to the DMPK gene, the main symptom of which is muscle degenerative atrophy , mostly occurs in adults over the age of 30, the patient will be completely incapacitated by the age of 50-60; this indication has at least 10,000 patients in China, and about 40,000 patients are known in the United States; DM1 No drug has been approved yet, and Avidity’s drug for this indication has entered clinical research and has been administered to more than 30 patients so far.
The incidence of DMD indications is similar in China and the United States, and it is estimated that there will be 61,000 patients in China; four drugs have been approved, of which Sarepta in the United States occupies three drugs, and the sales of these three drugs in Q2 in 2022 $211 million. In addition, there are many types of DMD genetic mutations, and the existing exon skipping therapy has limited efficacy, and there is still a huge room for development. At present, there are no drugs approved for marketing in China for the two indications of DM1 and DMD.
In order to further enhance the ability of original innovation, Jiajin Bio has built a research and development center of 1000+ square meters to speed up the efficiency of drug efficacy verification; and established cooperative relations with Fudan University, Shanghai Institute of Materia Medica, Chinese Academy of Sciences and other university research institutes, as well as first-line CRO enterprises. At present, Jiajin Bio is expanding its team and welcomes professionals who are willing to work on the research and development of small nucleic acid conjugated drugs .
It is understood that the current global research and development of small nucleic acid conjugated drug companies mainly include Avidity Biosciences, Dyne Therapeutics, Denali Therapeutics and Gennao Bio. This combination of innovative therapy is expected to bring differentiated therapy for muscle, tumor, and central nervous system diseases.
Investor’s View
Sun Linghao, managing director of Matrix Partners, said: We have been paying attention to the platform technology innovation of new drug forms, especially in the field of oligonucleotides. How to establish a safe and effective delivery platform to achieve non-liver targeting has always been the focus of the industry. Not long after its establishment, Jiajin Bio has built an excellent delivery platform and small nucleic acid drug research and development team, and is actively exploring small nucleic acid coupling technology and high-throughput screening of antibodies/peptides. We look forward to continuing to support the company in completing platform validation in the future and advancing multiple pipelines into the clinical stage.
Dr. Xie Da, vice president of Fengrui Capital, said: New drug forms have always been an important force in promoting the development of the biopharmaceutical industry. In recent years, the successful and rapid development of antibody-drug conjugates and small nucleic acid drugs in different disease fields has brought more possibilities and development space for new forms of drug conjugates in cross fields. The founding team of Jiajin Bio has rich experience in the pharmaceutical industry and biopharmaceutical investment. We are optimistic that Jiajin Bio’s market judgment, technical understanding and research and development of new and efficient conjugated drugs will provide new opportunities to overcome diseases such as rare diseases.
media coverage
36Kr Invests in China Net Venture State
Related events
- Jiajin Bio completed 45 million yuan angel round financing2022-11-09
- Youjia Bio completed the A+ round of financing of tens of millions of yuan2022-09-23
- Angtuo Bio completed nearly 100 million yuan seed round investment2022-09-19
- Targeted telomere gene therapy development company Yufang Bio has completed an angel round of financing of tens of millions of yuan2022-08-23
- RiboBio completes $40 million E1 round of financing2022-07-29
This article is reprinted from: https://readhub.cn/topic/8kNiPXKeHBb
This site is for inclusion only, and the copyright belongs to the original author.