Original link: https://kaopubear.top/blog/2022-12-17-newsletter-pro-2022-precision-oncology/
This article is a trial version of the content of the Xiongyan Xiongyu email newsletter membership plan . For the full content, please click on the newsletter homepage to subscribe and view it.
written in front
Hello, I am a bear who thinks about problems. This content is exclusive to readers who participate in the membership plan. Thank you for supporting my continuous creation through the membership plan. Zhanxinjia~
Contents of this issue:
Based on two articles published in Cancer Discovery in December, we will review the progress of precision tumor therapy in 2022 from the perspective of OncoKB’s new biomarker and FDA drug approval.
Next notice:
As I have summarized in this article , the past two decades of cancer research have given us a wealth of new targeted drugs. Although these drugs can be very effective initially, resistance to monotherapy remains a major challenge.
In the face of drug resistance, drug combination can sometimes help overcome drug resistance, but the number of drug combinations has far exceeded the level that can be used for clinical trials. In addition, the economic burden on patients and toxic side effects need to be considered comprehensively. Therefore, we need to identify potentially effective drug combinations based on an understanding of the molecular mechanisms involved in drug resistance. Relevant content, the next membership plan will study with you.
Well, the content of this issue has officially begun.
Literature information


Rosen, Ezra, Alexander Drilon, and Debyani Chakravarty. 2022. “Precision Oncology: 2022 in Review.” Cancer Discovery 12 (12): 2747–53. https://doi.org/10.1158/2159-8290.CD-22 -1154 .
Duke, Elizabeth S., Michael J. Fusco, Patrick DeMoss, Asma Dilawari, Gulsum E. Pamuk, Jessica Boehmer, Bronwyn Mixter, Kirsten B. Goldberg, Paul Kluetz, and Richard Pazdur. 2022. “Highlights of FDA Oncology Approvals in 2022 : Tissue-Agnostic Indications, Dosage Optimization, and Diversity in Drug Development.” Cancer Discovery 12 (12): 2739–46. https://doi.org/10.1158/2159-8290.CD-22-1185 .
about the author
The three authors of Precision Oncology: 2022 in Review are from MSKCC, and the corresponding author Debyani Chakravarty has cooperated with many doctors as a molecular geneticist and participated in the publication of many “landscape” type articles in MSKCC, such as:
- Oncogenic signaling pathways in the cancer genome atlas
- The immune landscape of cancer
- Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients
- Pathogenic germline variants in 10,389 adult cancers
If you are interested in this kind of article, you can search for articles signed by her.
Regarding this type of article, I just wrote a Foundation Medicine 420,000-case sample library a few days ago. I analyzed the mutation characteristics of KRAS pan-cancer species and came to these conclusions . There will be corresponding topics for us to study together.
Highlights of FDA Oncology Approvals in 2022 Elizabeth Duke, the corresponding author of this article, graduated from Harvard Medical School and joined the FDA Office of Oncologic Diseases (OOD) in August 2020 to participate in solid tumor and hematological tumor-related drugs and biological treatments Oversight, approval and oversight of method development.
About OncoKb Mutation Rating
Precision Oncology: 2022 in Review This article is written by the MSKCC team. The selected entry point of view is based on the mutation rating content updated before October 28, 2022 in OncoKB.

In OncoKB, annotation ratings for mutations are divided into four levels:
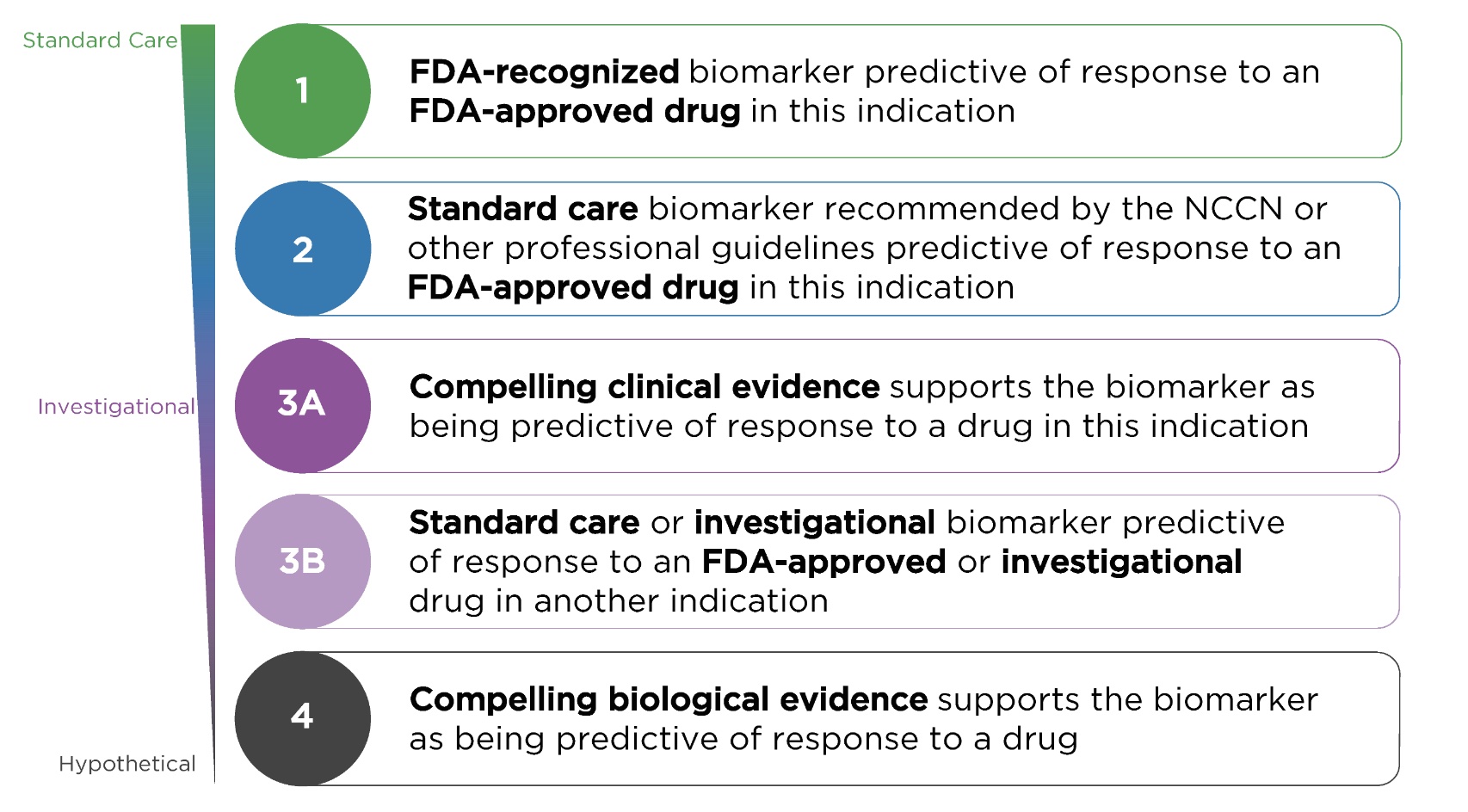
- Level 1: Related to FDA-approved standard treatment drugs. FDA-recognized biomarker predictive of response to an FDA-approved drug in this indication
- Level 2: related to standard treatment recommended by NCCN or other expert consensus. Standard care biomarker recommended by the NCCN or other expert panels predictive of response to an FDA-approved drug in this indication
- Level 3: Cancer-specific mutations that are considered predictive of clinical benefit in stage III or compelling stage I/II. Compelling clinical evidence supports the biomarker as being predictive of response to a drug in this indication but neither biomarker and drug are standard of care
- Level 4: Biomarkers that have been validated in preclinical models to predict response to targeted therapy. Compelling biological evidence supports the biomarker as being predictive of response to a drug but neither biomarker and drug are standard of care
Simply put, category 1 is a biomarker approved by the FDA (Osimertinib is used for EGFR L858R NSCLC), category 2 is the standard treatment biomarker recommended by the guidelines (crizotinib is used for NSCLC with MET amplification), category 3 It is a biomarker of clinical evidence level (Adagrasib is applied to patients with colorectal cancer KRAS G12C mutation), and a biomarker of category 4 is supported by biological evidence (ARID1A Truncating Mutations solid tumors use EZH2 inhibitor Tazemetostat)
According to OncoKB data as of October 28, 2022, the FDA has approved 6 indication treatments selected by specific biomarkers, and the NCCN guidelines have added 9 biomarker-specific indication treatments in 2022. Next, we summarize from the three dimensions of drug design, experimental research design and new biomarkers.
drug design
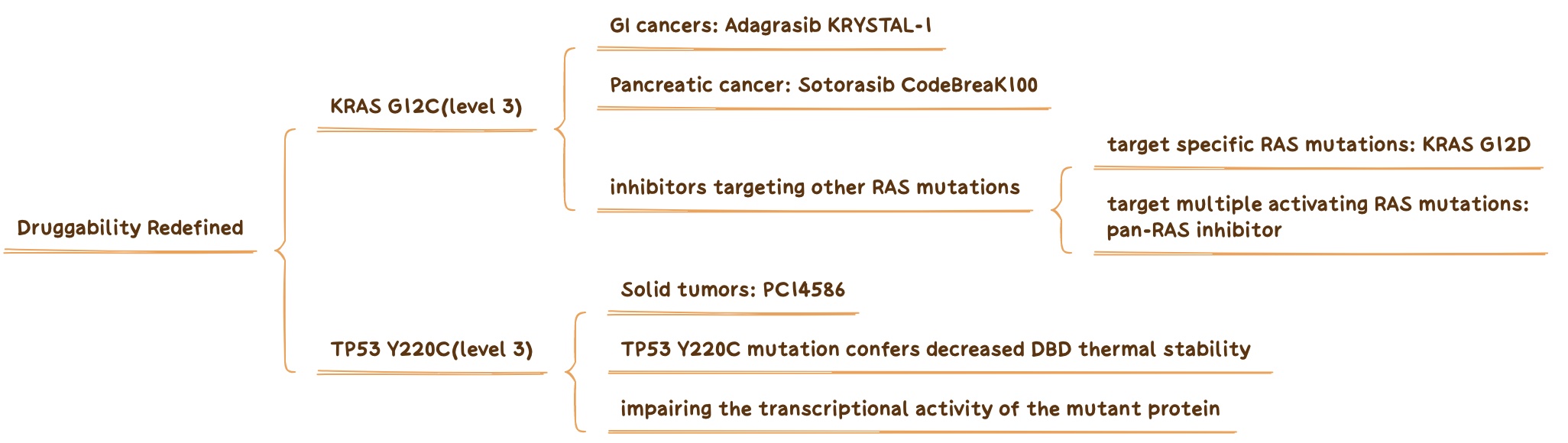
KRAS G12C and TP53 Y220C change the definition of a druggable target.
In 2022, the results of two important studies related to KRAS G12C, CodeBreaK100 and KRYSTAL-1, will be announced. The former study confirmed that the ORR of Sotorasib in pancreatic cancer was 21.1%, and the latter study confirmed that the ORR of Adagrasib in gastrointestinal cancer was 41%. Therefore, KRAS G12C has also obtained a new level3 rating for the corresponding cancer type in OncoKB.
In addition to the hottest mutation G12C, we should also pay attention to other pan-RAS inhibitors such as G12A/D/R that can target multiple activating RAS mutations (such as RMC-6236 ) in the next few years.
TP53 is the most commonly mutated gene in cancer, and until recently mutant p53 was thought to be drug-resistant. As a tumor suppressor and transcription factor, p53 is involved in the regulation of DNA repair and apoptosis. A heterozygous mutation in its DNA-binding domain produces a mutein that fails to function as a transcription factor. Mutant P53 can bind and block the activity of wild-type P53. The rare TP53 Y220C mutation can lead to decreased stability of the DNA-binding domain and decreased transcriptional activity of the mutant protein.
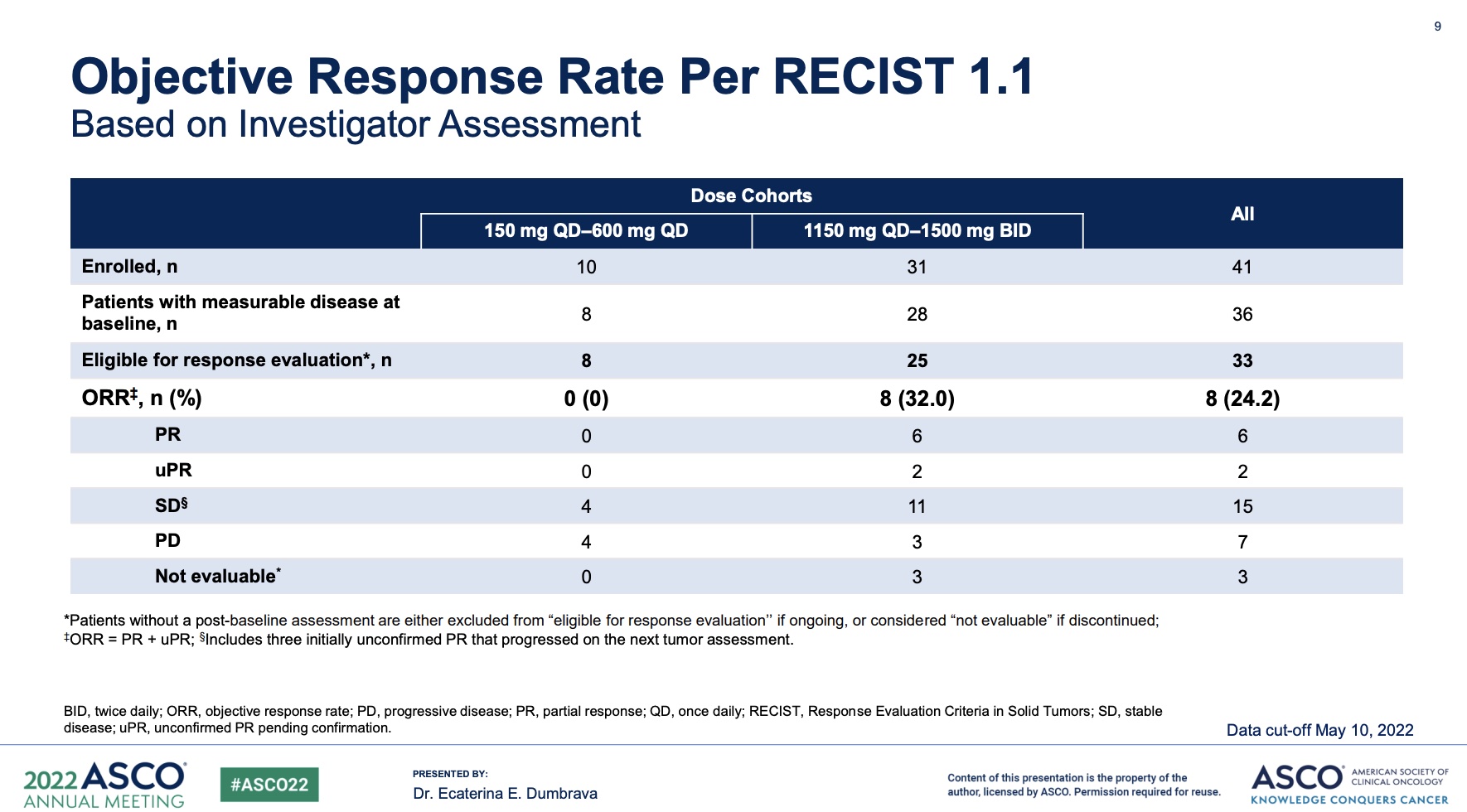
As presented at the 2022 ASCO Annual Meeting, patients with solid tumors harboring the TP53 Y220C mutation were treated with the targeted drug PC14586 in a basket trial, with six confirmed PRs among 33 evaluable patients.

A new generation of small molecule inhibitors is still being iterated.
In 2022, there will still be some new TKIs that will continue to solve various problems such as drug resistance, penetration of the blood-brain barrier, and increased tolerance.

 Trial end
Trial end 

The remainder of the content is exclusive to readers participating in the membership program
You are welcome to support my continuous creation through the membership plan
If you are interested in this article, please subscribe to my “Xiong Yan Xiong Yu” member newsletter via email or WeChat. I will share with you the latest industry research progress in the field of tumor biomedicine and what I think and learn. If you want , click this link to subscribe for free. 
This article is transferred from: https://kaopubear.top/blog/2022-12-17-newsletter-pro-2022-precision-oncology/
This site is only for collection, and the copyright belongs to the original author.