In the zinc-air battery, oxygen in the air is used as the positive active material, zinc is used as the negative electrode, and ammonium chloride or caustic alkali solution is used as the electrolyte.
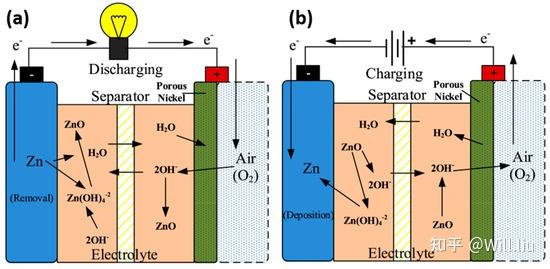
negative electrode:
Zn+2OH–→ZnO+H2O+2e–
positive electrode:
O2+2H2O+4e–→4OH–
comprehensive:
2Zn+O2→2ZnO
Not only the zinc-air battery research has won awards, but the four basic metal-air batteries have won various awards.
Aluminum-air battery, magnesium-air battery, zinc-air battery, lithium-air battery.
In principle, metal-air batteries are all fuel cells that use metal as a material and undergo a redox reaction with oxygen (air) to produce electrical energy. In layman’s terms, the metal negative electrode is used as an energy source, and the positive electrode inhales air as a tool to convert energy.
At present, the cathode materials of mainstream lithium power batteries are ternary and lithium iron phosphate materials, and the cathode materials are carbon-based materials such as graphite. The combined cost of the two accounts for more than half of the entire cell.
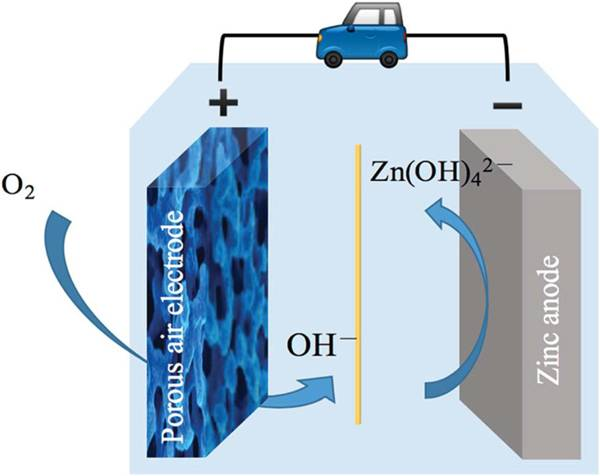
Taking zinc-air batteries as an example, zinc is used as the negative electrode, not a precious metal. It is rich in global resources (China has the largest storage capacity), and it is of high quality and low price, while the positive electrode material does not cost money and consumes oxygen in the air.
Advantages of metal-air batteries:
1. The energy density of the battery is high . The theoretical energy density of the zinc-air battery has reached about 1350wh/kg, and the energy density in some applications has reached 200-300wh/kg, which is actually close to the energy density of the ternary lithium battery. the limit theoretical value.
2. Safe and environmentally friendly. Zinc metal is not toxic, nor are other components in the battery flammable. This makes it possible that even if the zinc-air battery is internally short-circuited, the electrolyte will not burn or explode after leaking (corrosive), and the product of the battery, zinc oxide, is basically non-polluting and harmless to the environment.
3. Stable performance and stable discharge curve . The redox reaction of the positive and negative electrodes is not severe, and the change of voltage during discharge is small, and a long-term discharge platform will appear in the environment of 1.3V voltage.
4. The cost of positive and negative electrodes is low , one side is air, and the other side is zinc. The proven production of domestic zinc storage is the world’s largest, and the zinc reserves of other countries are not low, which makes the cost of zinc lower than that of other metals, which also enables low-cost operation in both mining and production. Especially compared with lithium batteries, both cobalt and lithium carbonate/lithium hydroxide materials in ternary lithium batteries are several times or even 10 times that of zinc materials.
But why do metal-air batteries have so many advantages, why are lithium batteries popular all over the world at this stage, and zinc batteries can only be used for small (miniature) commercial use, such as hearing aid batteries.
One of the important reasons is that the lithium power battery can be charged. When the power is exhausted, find a charging port and plug it in, and it will be fully revived after one or two hours.
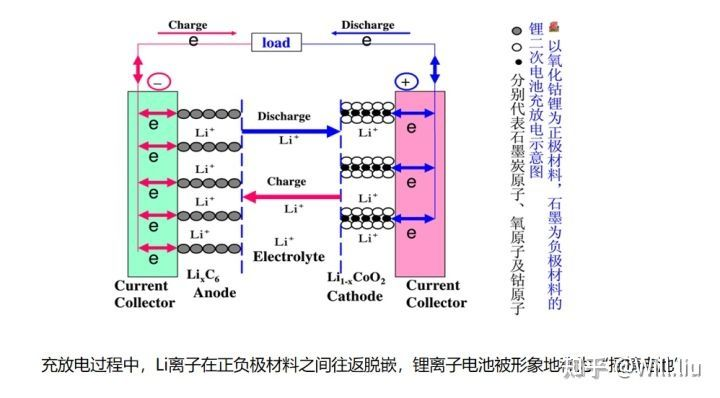
When charging, the lithium atoms on the positive electrode lose electrons and are oxidized into lithium ions (deintercalation) , then move in the electrolyte , run to the negative electrode, combine with the electrons flowing through the external power supply, and reduce to lithium atoms, embedded in the micropores of the negative carbon layer.
During discharge, it is the opposite process. The lithium atoms embedded in the carbon layer of the negative electrode lose electrons, are oxidized into lithium ions, return to the electrolyte, move to the positive electrode, combine with the electrons flowing through the load, and reduce again. became lithium atoms (intercalation) .
Therefore, during the charging and discharging process, lithium ions are deintercalated and deintercalated between the positive and negative electrode materials. This battery is also vividly called “rocking chair battery.”
In the traditional zinc-air battery, zinc turns into zinc oxide and releases electricity, which is a one-way street, one-time transaction, can only be discharged and not rechargeable, and belongs to a disposable battery. After all the zinc is consumed, the zinc-air battery is dead, and it is necessary to open the battery and replace the zinc (supplementary electrolyte) before it can be re-discharged.

For this reason, the current zinc-air battery is embarrassing, although it is loaded on the car, it can run the car for hundreds of kilometers. But the zinc oxidation is over, and it cannot be plugged in and charged like a lithium battery.
Either replace the whole zinc-air battery pack, or drag the battery pack out on the spot, change the zinc (supplement the electrolyte), and load it on the car.

There is an old saying that I don’t know if it’s inappropriate to say that the price of tofu is basically the process. Although the principle of zinc-air battery is simple, the raw materials are cheap, and the cost is half cheaper than that of lithium power battery, it can’t stand every two or three hundred kilometers. The time cost and labor cost caused by having to replace the battery are wasted.
In addition, zinc-air batteries have a congenital problem, that is, the air tightness is extremely poor. Because the discharge principle of zinc-air battery relies on the reaction between oxygen and zinc in the air, not only the battery cannot be sealed, but also the battery shell must have multiple air holes for air to enter (high ventilation requirements), whether CO2 enters the electrolyte to produce carbonic acid Whether the water vapor enters the battery to dilute the electrolyte (or the moisture of the electrolyte evaporates rapidly at high temperature) will seriously affect the discharge energy efficiency of the battery itself.
And this also brings another regional problem, that is, the low-oxygen environment cannot go, because it will cause the reaction to weaken and the power to drop.
In addition, zinc-air batteries have high energy density but low specific power for a number of reasons.
One is the air diffusion electrode catalyst, and the other is because the current zinc particle structure makes the internal resistance of the battery increase in the moving state, and the vibration of the vehicle causes the contact resistance between the zinc particles to increase.
The characteristics of this zinc-air battery are that the electric capacity is large, but it cannot be discharged when there is electricity, which is exactly the opposite of the characteristics of the supercapacitor mentioned in my previous answer. A supercapacitor is a battery with low energy density, but high specific power, and the discharge is fierce.
The low power of the zinc-air battery will make the vehicle weak when it needs high power output, such as acceleration and climbing, which requires high-power discharge. Just to give you face, as for overtaking, I advise you to drive safely…
In addition, because the zinc-air battery is a combination of strong corrosive electrolyte (strong alkaline) and pores, when the car vibrates (which cannot be avoided), the electrolyte may leak out and corrode the casing. And once the insulator of the casing is damaged, electricity will leak out… One is that the electricity will drop rapidly while running, and the other will cause danger when changing the battery…
Finally, the recyclable solution of zinc-air battery is to solve the irreversible phenomenon of zinc oxidation.
In January 2021, the first “Science” related to zinc-air batteries was published.

The relevant information is placed in the reference materials, and the analysis is very detailed. I will not repeat it here. If you are interested, you can read it.
Next, in September 2021, the US energy storage company EOS claimed that the developed zinc-air battery can achieve 2,700 cycles of charge and discharge. Extend battery life with improved electrolyte chemistry and battery design. Their small test proved that a 0.3 kW battery can achieve 2,700 charge and discharge cycles without performance degradation. Its ultimate goal is to achieve 10,000 cycles, and the product will maintain a 30-year life in grid energy storage.
So maybe 10 years later, it is not impossible for metal-air batteries to replace lithium power batteries as the driving force for family car travel.
(Picture source network, invade and delete!)
References:
Source: Zhihu www.zhihu.com
Author: Will.liu
[Zhihu Daily] The choice of tens of millions of users, to be a big cow to share new things in the circle of friends.
click to download
There are 9 more answers to this question, see all.
Further reading:
This article is reproduced from: http://www.zhihu.com/question/266539528/answer/2521887430?utm_campaign=rss&utm_medium=rss&utm_source=rss&utm_content=title
This site is for inclusion only, and the copyright belongs to the original author.