Disclaimer: This article is only a record of personal investment thinking and does not constitute investment advice.
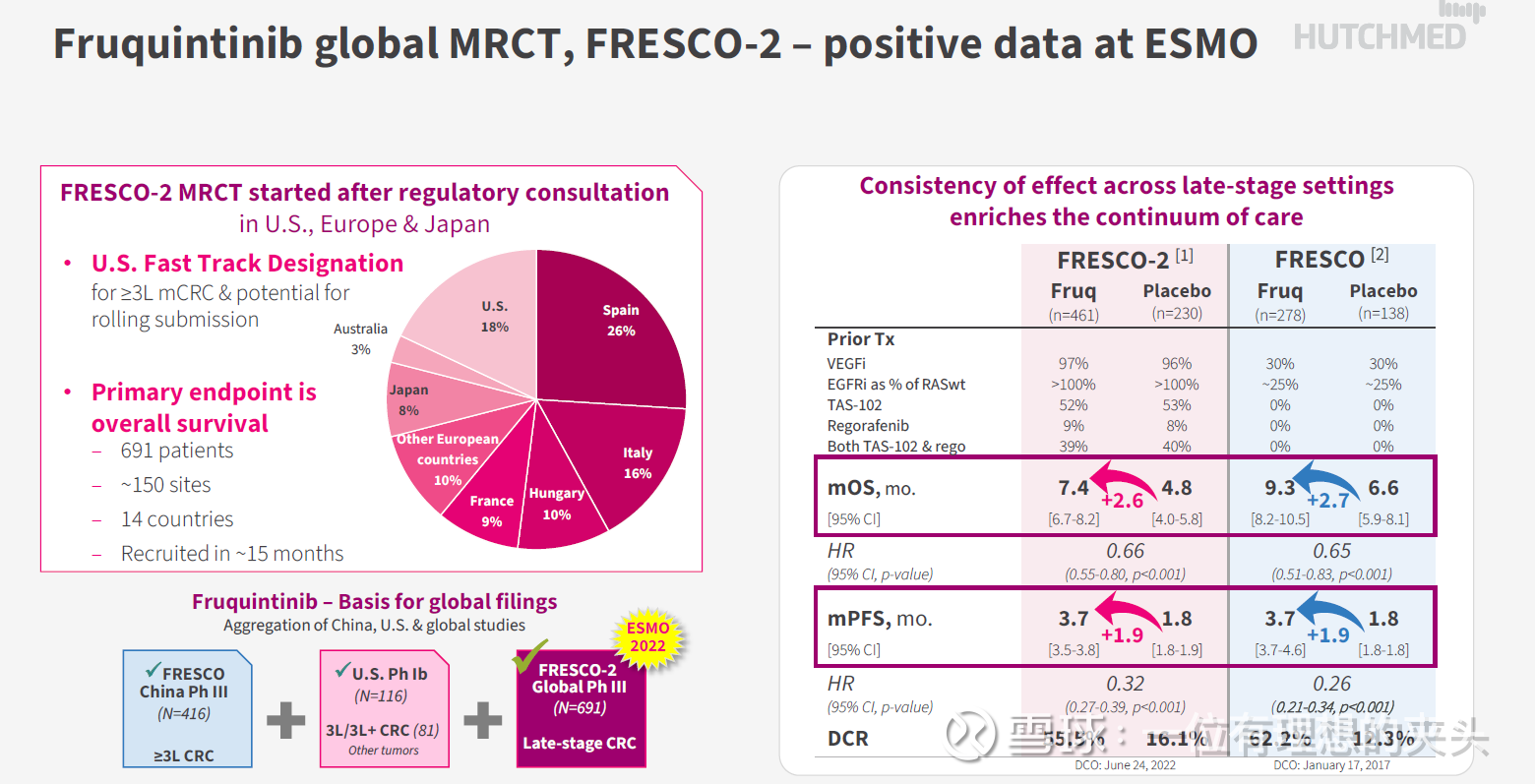
Chi-Med is a target that I have always adhered to, and it is also a target that I am very optimistic about. Chi-Med announced on January 23, 2023 that Chi-Med announced that its subsidiary Hutchison Whampoa Pharmaceuticals (Shanghai) Co., Ltd. had reached an exclusive licensing agreement with a subsidiary of Takeda Pharmaceutical Company Limited (Takeda Pharmaceuticals), except in mainland China and Hong Kong. Further advance the development, commercialization and production of fruquintinib globally outside of Macau. Fruquintinib is marketed by Chi-Med in Mainland China, Hong Kong and Macau. Hutchison Pharmaceuticals Limited will receive payments totaling US$1.13 billion, including an upfront payment of US$400 million at closing, potential regulatory registration, development and commercial milestone payments, plus net sales-based payments Royalties. In fact, this overseas authorization is based on the results of the FRESCO-2 international multi-center phase III clinical trial of fruquintinib for the treatment of patients with refractory metastatic colorectal cancer, and achieved the main result of OS.
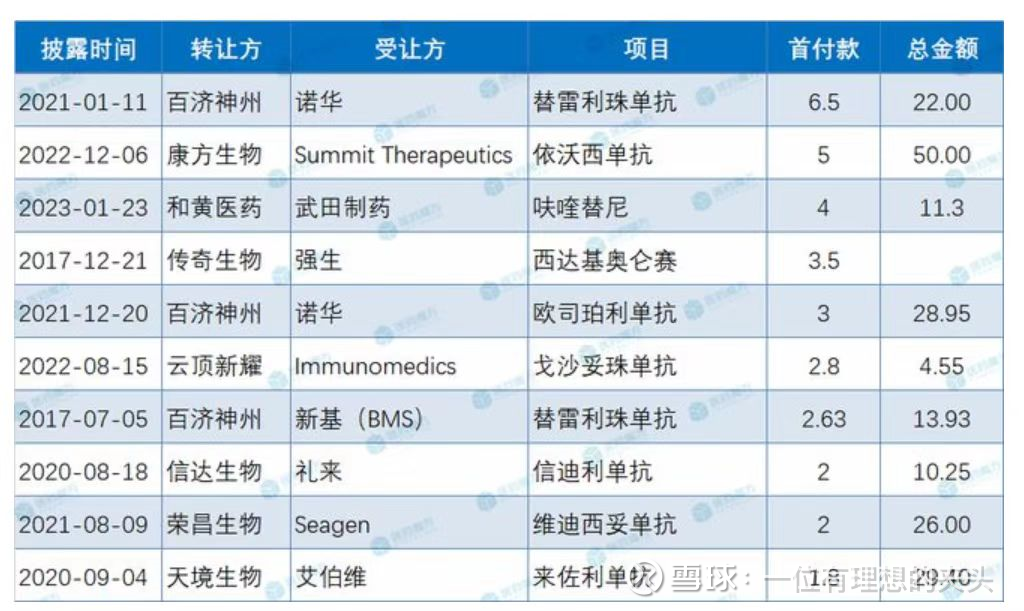
Overseas BD down payment
Up to now, Chi-Med’s fruquintinib’s overseas authorized down payment ranks third, ranking first among small-molecule drugs, even higher than Legend Bio’s licensing of Johnson & Johnson’s Cedargiolund and Rongchang Bio Vidicumumab (RC48) for Rongchang Biotech.
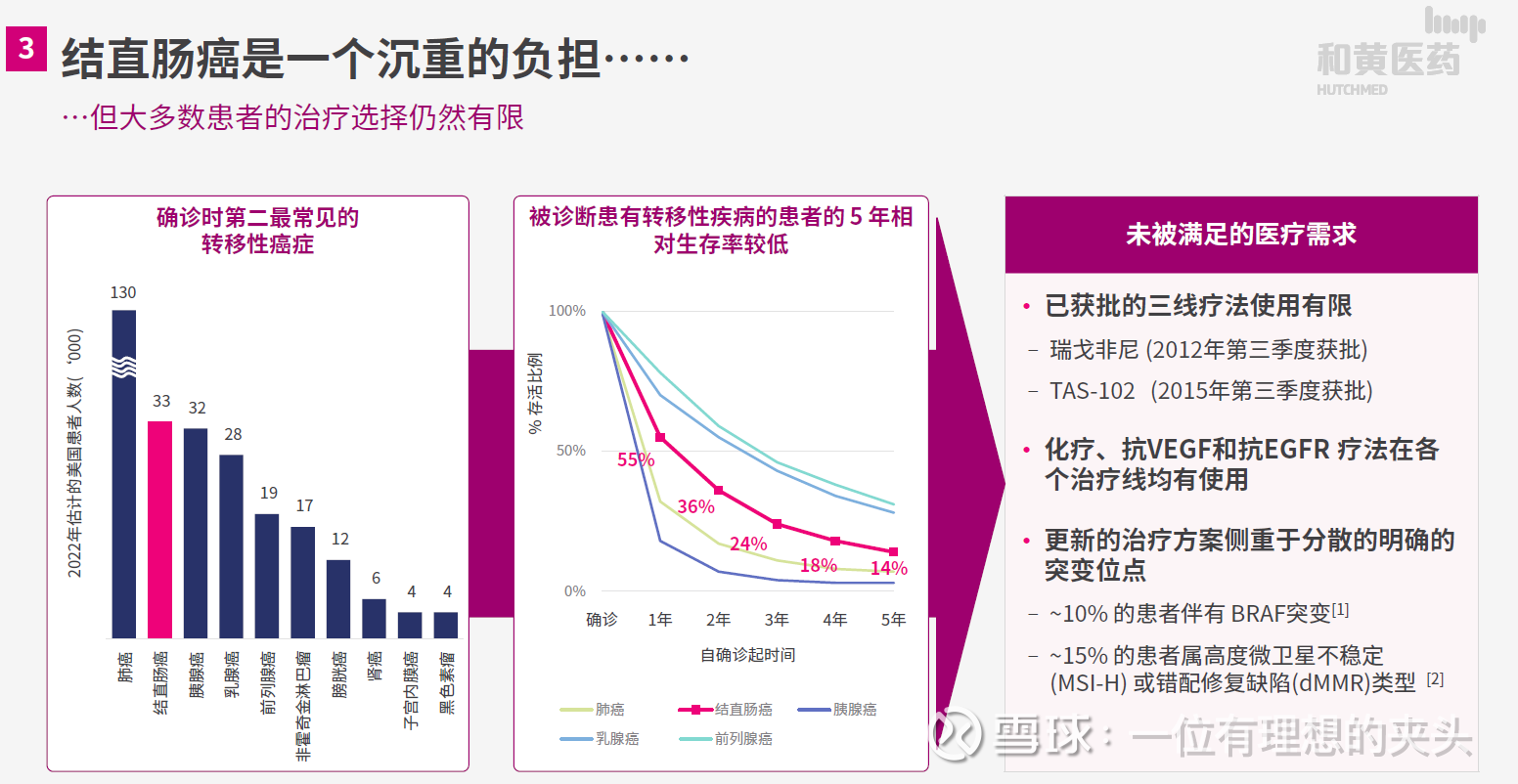
From this perspective, Takeda Pharmaceutical is very optimistic about Chi-Med’s fruquintinib as a third-line treatment for metastatic refractory colorectal cancer. The incidence of colorectal cancer is very high, and the mortality rate is extremely high, especially the treatment options for metastatic refractory colorectal cancer are limited. In China, the market share of regorafenib has also been surpassed by fruquintinib. As of the 2022 semi-annual report, fruquintinib has already occupied 43% of the market share. According to the previous communication, the global market potential of 3LCRC is about 3 billion to 4 billion US dollars, so the overseas market of fruquintinib is still quite large, and it depends on Takeda Pharmaceutical to develop it.
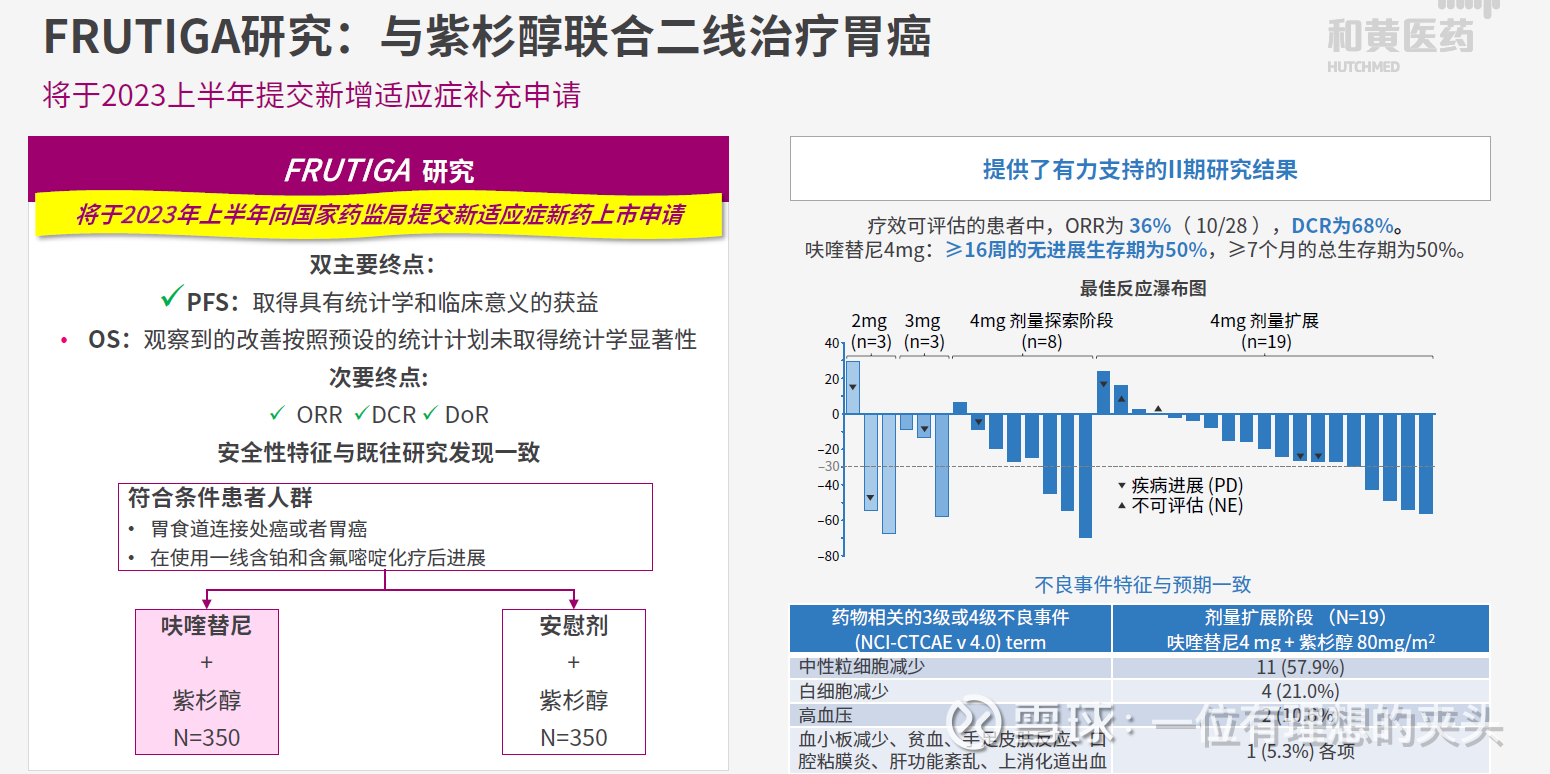
Fruquintinib may add second-line gastric cancer indication
The FRUTIGA study was designed to evaluate the combination of fruquintinib and paclitaxel in patients with advanced gastric cancer or gastroesophageal junction (GEJ) adenocarcinoma. The study included 703 Chinese patients. Chi-Med intends to submit an NDA for this indication in 2023. If approved, the market value of fruquintinib will increase significantly.
Combination with fruquintinib
Phase II/III study of fruquintinib combined with Innovent’s PD1 (sintilimab) in the treatment of advanced renal cell carcinoma. The primary endpoint of the study is PFS, and the secondary endpoints include OS, DCR, etc. Five immune-oncology combination therapies have been approved in the United States for the first-line treatment of advanced renal cell carcinoma, but this indication has not been approved in China at present, so this indication is also to meet medical needs. The previous phase IB/II data It’s still relatively good. Looking forward to better data in phase II/III, the sales of fruquintinib will inevitably increase.
2023 Catalyst
In addition to the above-mentioned overseas BD of fruquintinib and the submission of NDA by FRUTIGA, the data of Solepineb and Andilised were also read out in the second half of the year, and the indications of these two hematoma products have obtained breakthrough treatment drug.
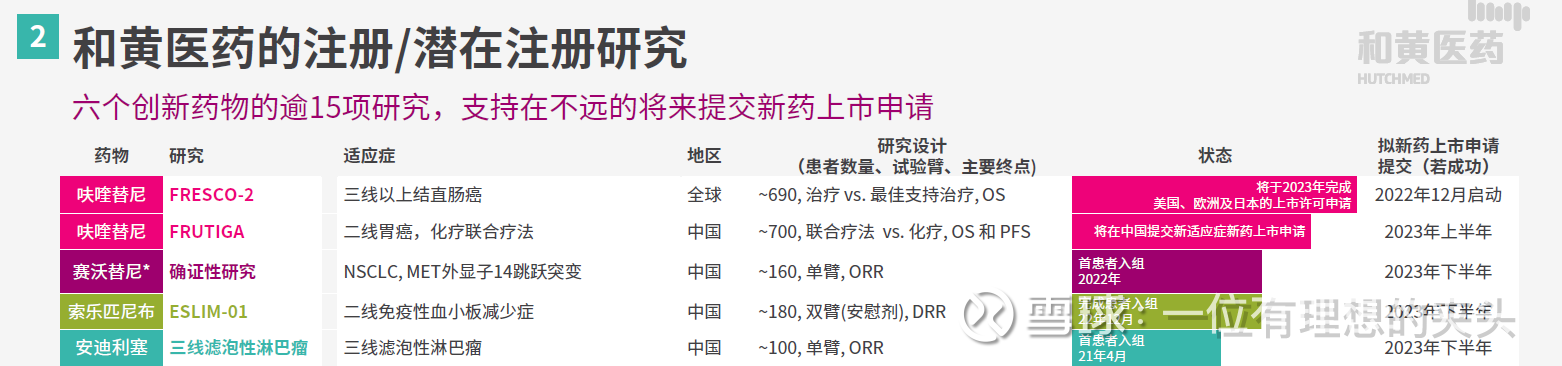
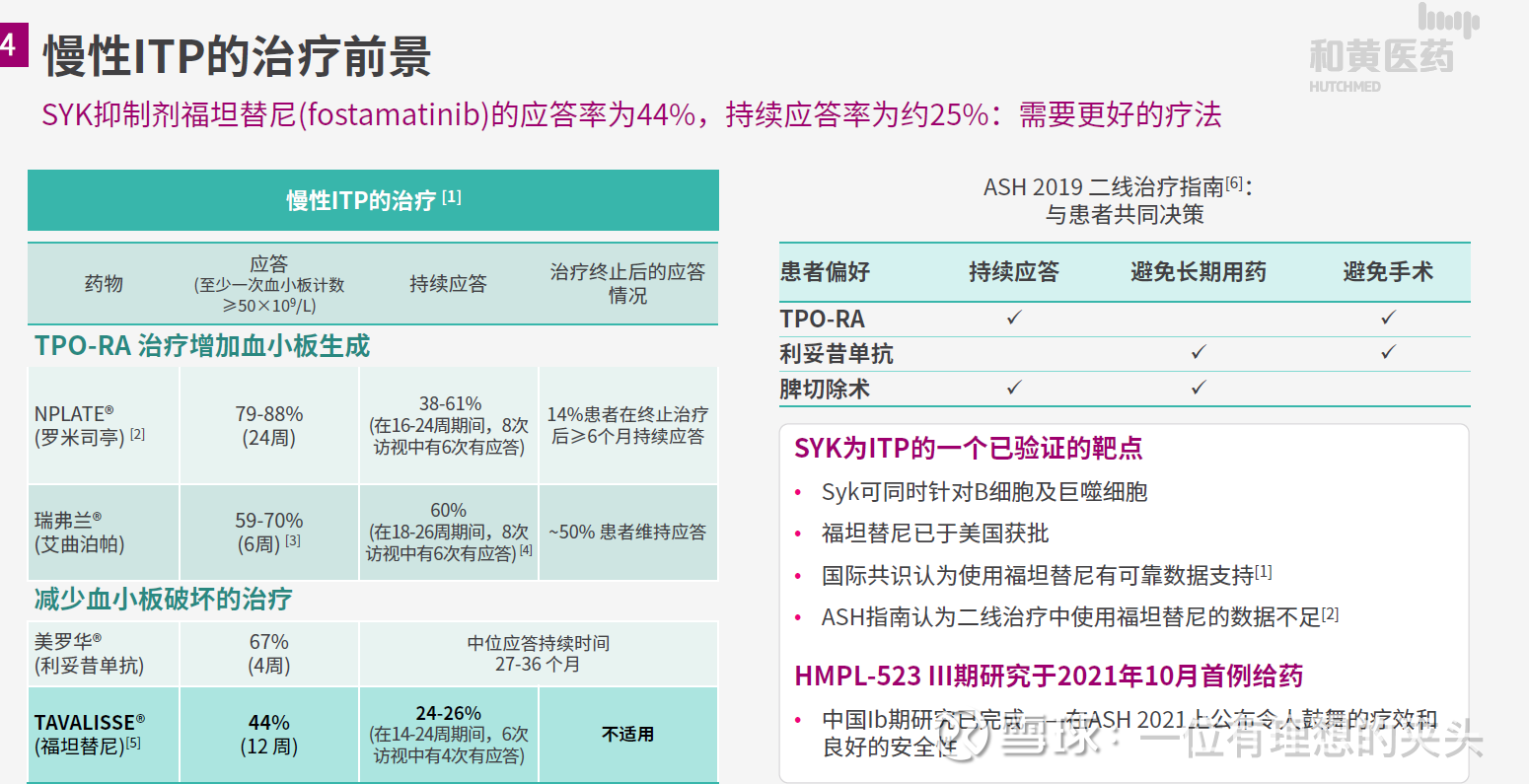
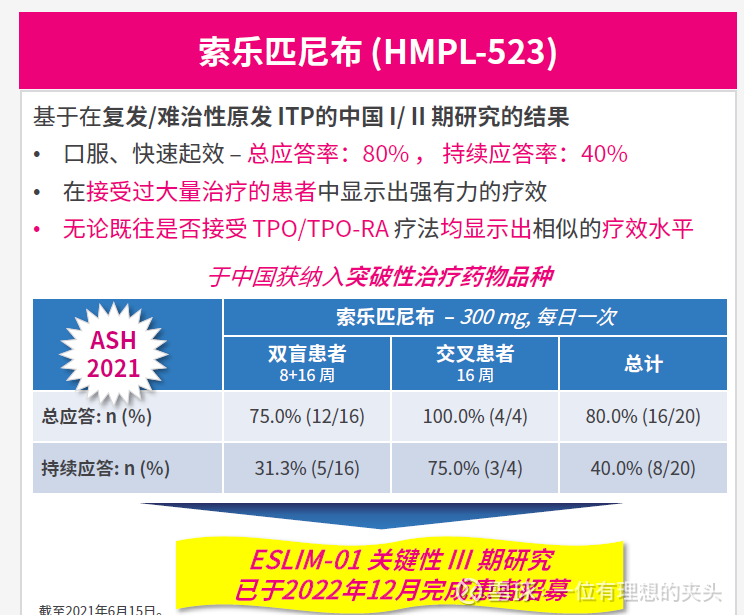
The ESLIM-01 China phase III clinical study of solotinib for the treatment of primary immune thrombocytopenia will complete enrollment on December 31, 2022. The primary endpoint of the study was the sustained response rate, and the secondary and exploratory endpoints were ORR, TEAE, and improvement in quality of life. Solepineb is likely to be a me better product of SKY target inhibitors, and the top-line results of the ESLIM-01 study are expected to be announced in the second half of 2023.
The results of the treatment of the third-line FL indication of Andilised will also be announced in the second half of 2023, and this indication has been granted a breakthrough therapy by the CDE. If successful, the NDA will also be submitted in the second half of 2023. These are the catalysts of Chi-Med.
Extremely Undervalued Chi-Med
Chi-Med currently has US$826 million in cash in its account, and will get US$400 million after the completion of the plan. Adding about 10 billion to 12 billion of the Musk Baoxin Pill assets, the current market value is 23.6 billion Hong Kong dollars, while other pipelines have not given valuations. At the same time, cervotinib may be a large single product with a sales peak of over 5 billion. The two hematological tumor products, Andilise and Solonibib, also have great potential. See if the pipeline of Hutchison Medicine below can Give a valuation? Chi-Med will not reduce its position until its stock price reaches HK$56. Moreover, with the increase in market liquidity, certain innovative pharmaceutical companies should be given a certain premium for overseas licensing to Takeda.
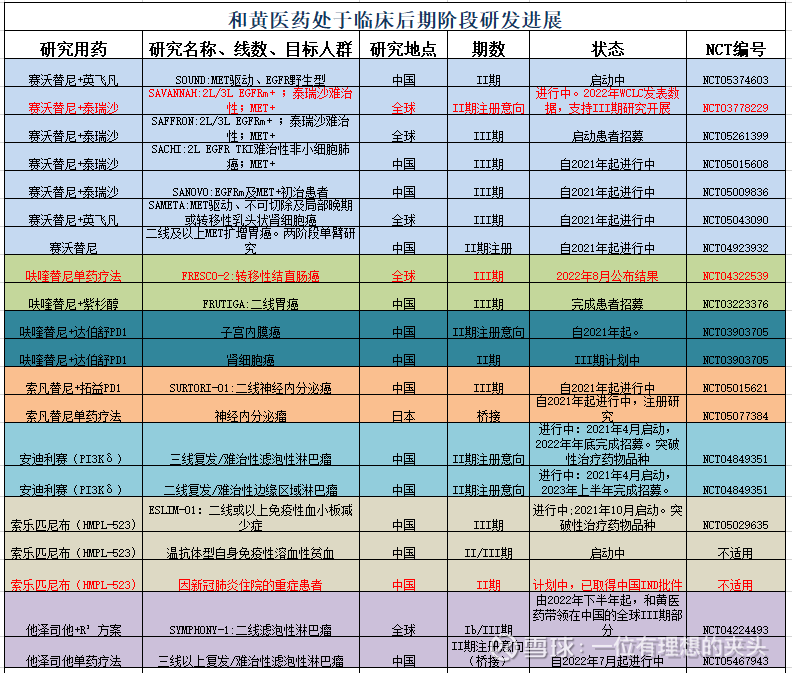
In short, the value of Chi-Med is accompanied by the authorization to Takeda Pharmaceutical, and institutions will increasingly tap the opportunities of Chi-Med. Chi-Med is a small “Baekje China”, with a lot of cash, a mature business team, a very well-qualified management team, and differentiated products .

But now there are not only small molecules, but also double antibodies and monoclonal antibodies, and also authorized the early products to Chuangxiang Bio, which is responsible for the direction of autoimmunity, and the tumor direction and Huangyao Pharmaceutical. Such an excellent company Don’t get out of the car easily. $Baekje Shenzhou(06160)$ $Rongchang Biology(SH688331)$ @今日话题@雪球创创中心
There are 19 discussions on this topic in Xueqiu, click to view.
Snowball is an investor social network where smart investors are all here.
Click to download Xueqiu mobile client http://xueqiu.com/xz ]]>
This article is transferred from: http://xueqiu.com/6625427821/240627255
This site is only for collection, and the copyright belongs to the original author.